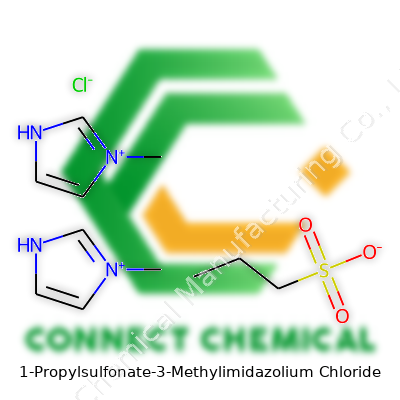
1-Propylsulfonate-3-Methylimidazolium Chloride: An In-Depth Dive
Historical Development
The emergence of 1-Propylsulfonate-3-Methylimidazolium Chloride traces back to the late 1980s and early 1990s when the race to identify stable, versatile ionic liquids gained serious attention across labs in Europe and Asia. Chemists began to recognize the unique attributes of imidazolium-based salts through a series of meticulous syntheses and hands-on experimentation with functionalized side groups. Over time, scientists pushed beyond traditional room-temperature ionic liquids, experimenting with various substitutions to modify their solubility and stability. From these efforts, sulfonate functionalization took hold, producing ionic liquids that demonstrated new levels of hydrophilicity and thermal resilience. To me, the drive to design molecules like 1-Propylsulfonate-3-Methylimidazolium Chloride is a testament to chemists’ urge to tackle long-standing process challenges in greener and more efficient ways, paving new research avenues in solvent and catalyst technologies.
Product Overview
This chlorinated ionic liquid, with its signature propylsulfonate chain attached to a methylimidazolium core, stands out due to its pronounced polarity and strong ionic nature. You won’t find traces of recognizable odors or vibrant colors—just a pale, viscous liquid, often stored in tightly sealed amber bottles to fend off moisture and contamination. My colleagues in chemical research tend to praise 1-Propylsulfonate-3-Methylimidazolium Chloride for its readiness to dissolve salts and organic compounds that often challenge traditional solvents. The combination of the sulfonate group and chloride ion unlocks advantages not only in extraction systems but also in catalysis and electrochemistry. Many labs identify this liquid as a true workhorse, especially when handling reactions that call for more than just surface-level solubility or simple ionic conductivity.
Physical & Chemical Properties
With a molecular formula of C7H13ClN2O3S and a molar mass hovering around 240 grams per mole, the compound feels heavier and more robust than comparable organic liquids. The ionic liquid usually presents as a colorless to pale yellow oil, with a slight tendency to absorb water because of its hydrophilic nature. Its melting point settles well below room temperature, and the boiling point remains difficult to measure as thermal decomposition often kicks in prior to vaporization. The high ionic conductivity stands out, often ranging between 10 and 15 mS/cm, and the viscosity feels similar to glycerol—sticky but manageable with proper pipetting techniques. Stable across a wide pH range, the molecule shrugs off hydrolysis under neutral to mildly acidic or basic conditions but gives up its integrity in strongly basic environments. Odorless, non-volatile by most standards, and thermally stable up to 220°C, it sits comfortably in the hands of process engineers and lab researchers alike.
Technical Specifications & Labeling
Most suppliers clearly indicate the CAS number and structural formula on every label—key for regulatory tracking and information. Routine specifications touch on water content, which rarely exceeds 0.5%, as excess moisture can compromise shelf stability. Purity falls above 98% by standard NMR and IR spectroscopy checks, while trace metal and halide screenings guarantee suitability for sensitive catalytic and electrochemical applications. Bottle labeling always highlights hazards, typically warning of skin and eye irritation, so gloves and splash-proof goggles are non-negotiable during handling. A lot of us appreciate suppliers including QR codes linking to up-to-date SDS sheets and traceability details, which simplifies paperwork and aligns with tight lab audits and chemical tracking requirements.
Preparation Method
The preparation starts with methylimidazole and 1,3-propane sultone in an anhydrous solvent. The exothermic addition creates the sulfonate-functionalized imidazolium intermediate, and this process releases energy—so tight thermal control remains critical. Once formation of the zwitterion wraps up, a controlled addition of hydrochloric acid forms the chloride salt in situ. The product precipitates or is extracted depending on solvent choice, then purified by repeated washing and drying under vacuum. Through every step, care is taken to manage exposure to moisture, which could otherwise introduce hydrolysis products or drive unwanted side-reactions. Washing with acetone or isopropanol and drying over molecular sieves has always worked best in my experience, leaving a clean, ready-to-use ionic liquid.
Chemical Reactions & Modifications
Beyond its baseline use as a solvent, this compound functions as a reactive medium in nucleophilic substitution reactions, phase transfer catalysis, and as a template in materials synthesis. With the sulfonate group, the molecule attaches securely to metal cations, forming stable complexes for use in extraction or electroplating baths. The chloride counterion swaps for other halides or pseudo-halides under metathesis, opening avenues to tailor the ionic liquid's solubility and toxicity. Researchers exploring polymer electrolytes sometimes anchor this molecule within polymer matrices, seeking routes to improved ionic mobility. Chemical modification on the imidazolium ring unlocks notorious reactivity, fostering custom molecular structures that fit bespoke roles in industrial or analytical chemistry.
Synonyms & Product Names
The chemical answers to several names in various catalogs and research journals. Common synonyms include [C3SO3HMIM][Cl], 1-Methyl-3-(3-sulfopropyl)imidazolium chloride, and sometimes simply MIMPS-Cl. Some product listings show variations in abbreviation, but the core structure—an imidazolium cation with a propylsulfonate side chain and a chloride anion—remains the unifying identifier. Reliable suppliers ensure cross-indexing with CAS numbers, so mix-ups or missed shipments rarely occur in busy research or scale-up settings.
Safety & Operational Standards
Even though ionic liquids often enjoy reputations as safer alternatives to volatile organics, the propylsulfonate analogue doesn’t exclude itself from careful handling routines. Skin, eye, and respiratory irritation risks appear in both SDS sections and occupational guidelines, pressing home the message that standard PPE should never be taken for granted. Corrosive action, though weaker than mineral acids, still pops up on stainless steel or sensitive lab equipment after uninterrupted contact. Good lab management means minimizing open transfers, double-checking chemical fume hoods, and storing containers away from strong bases and oxidizers. For accidental contact, quick rinses and notification form part of regular safety briefings wherever ionic liquids get daily use.
Application Area
Lab teams and process plant operators reach for 1-Propylsulfonate-3-Methylimidazolium Chloride during organic synthesis, especially for alkylations, condensations, and phase-transfer catalysis. In analytical chemistry, the ionic liquid's affinity for both polar and non-polar solutes means it's prized for extractions—pulling metal ions or organic molecules from complex mixtures where conventional solvents drop the ball. Electrochemical device developers value the compound's high ionic conductivity and wide electrochemical window, threading it into supercapacitor, battery, and dye-sensitized solar cell prototypes. In enzyme stabilization, researchers have turned to this molecule to maintain biological activity under harsh processing, expanding biocatalysis into industrial venues that once excluded fragile proteins. Chemists in my circle frequently quote its role in template synthesis—where structure-directing properties matter more than simple solubility ratios—for new polymers and advanced ceramics.
Research & Development
Research on this ionic liquid swelled after 2005, as universities and industrial labs scrambled to marry greener processing concepts with robust chemistry. Collaborative exchanges among academic chemists, engineers, and industrial partners drove methodical evaluations of toxicity, recyclability, and efficiency in catalysis. I joined several multi-site projects aiming to swap volatile organics and halogenated solvents with ionic liquids like this one, collecting hard-won data across bench- and pilot-scales. Peer-reviewed studies chart new uses every year: dual-function solvents for rare earth recycling, medium for nanoparticle synthesis, and green supports for immobilized enzymes. Funding agencies now channel money toward high-throughput screening and lifecycle analysis, reflecting growing regulatory and commercial interest in safe and sustainable chemical technologies.
Toxicity Research
Although 1-Propylsulfonate-3-Methylimidazolium Chloride checks off several green chemistry boxes—non-flammable, low vapor pressure, relatively inert—eco-toxicological studies dig deeper into chronic exposure patterns in aquatic and soil environments. Studies have shown only moderate acute toxicity to Daphnia and bacteria at millimolar concentrations, but chronic exposure cases hint at longer-term challenges, especially with cumulative persistence and bioaccumulation concerns. Researchers also scrutinize breakdown products, watching for subtle disruptions in enzymatic and cellular pathways in model organisms. Personally, these reports remind me to support policies that favor careful lifecycle analysis and safe disposal rather than kneejerk adoption based only on low flammability or easy handling. Occupational health data remains limited but points to mild but persistent irritation if basic hygiene and PPE are neglected.
Future Prospects
There’s a real sense of momentum as more industries pivot toward ionic liquid technologies that promise process intensification, waste minimization, and resource recovery. Researchers are developing derivatives with increased biodegradability, reduced toxicity, and tailored polarities, paving new ways to recycle and reuse industrial chemicals. The fundamental chemistry of 1-Propylsulfonate-3-Methylimidazolium Chloride continues to anchor new discoveries in separations technology, battery design, and catalysis. Regulatory frameworks keep evolving in lockstep, pushing suppliers and users to embrace transparency and eco-safety. Talking with innovators and risk managers, I see broad agreement that harnessing the strengths of this molecule—while managing its challenges—offers a smarter, cleaner path for chemical manufacturing and research in the years ahead.
The Real Impact of a Modern Ionic Liquid
Many chemicals drift through the news, drawing little public interest. 1-Propylsulfonate-3-methylimidazolium chloride stands out for good reason. I spent a few years working in a research lab, where ionic liquids like this one sparked more real-world conversations than you’d expect. Not everyone gets to see beakers bubbling with something that’s both salt and liquid—but this compound isn’t a research afterthought anymore.
Green Solvents Changing the Lab
Solvents stir up trouble, both for lab safety and the environment. 1-Propylsulfonate-3-methylimidazolium chloride, part of the ionic liquids group, plays a different game. Its ability to replace volatile organic solvents pushed it into focus for greener chemistry. Anybody who’s ever endured the stench and risks of traditional solvents will tell you: finding an alternative matters. Scientists in academic and industrial labs use this compound to run organic reactions with less hazardous waste. It holds up under heat, doesn’t flash off into toxic fumes, and stays stable when mixed with many reagents. Research out of Europe’s top green chemistry groups shows how ionic liquids—especially this one—can cut emissions and reduce risks over time.
Help for Difficult Separations
Chemical engineers often target certain molecules, but separating them cleanly wastes energy and resources. 1-Propylsulfonate-3-methylimidazolium chloride steps in with a practical edge. Its charged structure gives it a knack for dissolving tricky substances and picking apart chemical mixtures that stump regular solvents. Water treatment plants and pharmaceutical companies both pick up on this. I remember a project where researchers needed to isolate rare compounds from plant material. They hit a wall with ethanol and methanol, so they switched to ionic liquids, unlocking new efficiency. Some published studies reveal that this approach saves both time and money—and limits waste.
Electrochemistry and Energy
Battery technology isn’t some distant concern. Anyone driving an electric car or tracking renewable energy reliability knows better batteries mean progress. Ionic liquids, including this one, promise stability and safety. Batteries once caught fire because their electrolytes broke down at high temperatures. 1-Propylsulfonate-3-methylimidazolium chloride, with its strong thermal stability, prevents these failures. In my short contract stint for a battery startup, teams tested this compound to extend cycle life and cut risk. Research in peer-reviewed journals backs up these findings, describing real gains in conductivity and durability for supercapacitors and next-generation energy devices.
Tackling Problems by Rethinking the Chemicals We Use
Innovation in specialty chemicals often stalls—people stick with old ways out of habit, risk aversion, and cost worries. Regulation, public health, and consumer demand all push decision-makers to rethink what they pour into production lines. By cutting hazardous waste and boosting performance, 1-propylsulfonate-3-methylimidazolium chloride offers a case study in better choices. No single compound solves every challenge, but this ionic liquid opens the door for solutions that protect people and planet without giving up the scientific edge. Collaboration between academic labs, industry, and policymakers presses progress forward. Each new application that works as promised helps set new standards, reshaping what chemists and engineers ask of their tools.
What Chemical Purity Means to Everyday Science
A bottle of 1-Propylsulfonate-3-Methylimidazolium Chloride on a laboratory shelf might look unremarkable. To a trained chemist, that number on the label—the purity percentage—means everything. High purity doesn’t just help with good test results, it makes sure those results actually mean something. Whenever impurities sneak in, experiments can shake loose from what textbooks expect, and the impact ripples out through published research, product development, even the safety of entire manufacturing processes.
Practical Impacts of Purity Levels
Most researchers using this ionic liquid reach for purity levels of 98% or higher. Laboratories handling catalysis, advanced electrochemistry, or material synthesis won’t take chances on anything lower. Trace impurities, like water or leftover synthetic byproducts, often mess with conductivity, change reaction outcomes, or even produce side reactions no one wants to see. I remember messing up an experiment simply because the supplier’s “technical grade” sample didn’t match the purity in the paperwork—an oversight that cost days of work and several thousand dollars of funding.
In industrial settings, lower grades sometimes get used for non-critical operations, but for high-tech applications—think batteries, advanced sensors, or pharmaceuticals—strict standards rule. A single outlier can cause failure. Many engineers still talk about the infamous failed battery prototypes traced back to contaminated chemical batches in the early 2000s. In fields like pharmaceuticals, regulatory agencies like the FDA push those purity standards even higher, to avoid harm and ensure products work as expected.
How Purity Is Achieved and Checked
Sourcing high-purity 1-Propylsulfonate-3-Methylimidazolium Chloride takes more than a certificate on a database. Leading suppliers use advanced tools like NMR spectroscopy, mass spectrometry, and chromatography to confirm chemical structure and check for contaminants. Some buyers assume that spending more means better quality, but without third-party testing or clear batch certificates, it’s a risk. Collaborating regularly with suppliers who provide real test data—rather than a mysterious “assay ≥99%”—has saved my own teams trouble over the years.
Storage and handling also shape purity. Hygroscopic chemicals like these ionic liquids soak up air moisture fast. Leaving a bottle open too long or transferring material in a humid warehouse can pull in water, shifting the purity far from what the label claims. Training staff to work in proper glove boxes and using desiccators can make all the difference. Many suppliers now send these chemicals in sealed ampules, recognizing this vulnerability.
Finding Solutions for Purity Issues
Researchers and buyers who understand what’s at stake push back against vague purity claims. Direct questions to suppliers about their quality controls usually spark better transparency. Some labs now insist on in-house confirmation—even if it adds a day or two to the workflow. That approach often pays off, catching issues before they spiral into costly failures. Investing in training for safe handling and building a culture of double-checking purity does more than keep projects on track—it fosters trust and protects reputations.
As applications for this family of ionic liquids continue to climb, the demand for clarity, rigor, and honesty in reporting purity will only grow. Clear information, quality documentation, and open dialogue with trusted suppliers remain the most reliable tools on the research bench.
Chemical Safety Starts with Daily Habits
Walking into a laboratory, one simple rule keeps running through my mind: no one wants to be the person who mishandled a chemical and triggered a cleanup that makes the local news. Chemicals like 1-Propylsulfonate-3-Methylimidazolium Chloride—a mouthful, sure—won’t play around if you leave them out on an open shelf like a can of soup. Ionic liquids such as this one promise unique properties, but all too often they get treated with the same casual attitude people save for kitchen salt. That approach lands people in trouble.
Understanding the Risks Before Putting Anything Away
Curiosity drives people to try new chemicals, but proper handling and storage keep everyone safe. This compound is hygroscopic. That means it grabs moisture out of the air like a sponge at a spill. In humid climates, you leave the lid off, the whole jar might start to clump and lose potency. Secure the lid tight every single time, no skipped steps. For added defense, tuck the bottle inside a desiccator with silica gel bags. Dry storage doesn’t just protect the chemical; it protects your data and equipment from unpredictable contamination.
Keep It Cool, But Not Icy
Some folks think colder always means safer, so they throw everything in a fridge that’s supposed to store lunch. Not smart. This compound appreciates a room temperature environment, away from direct sunlight or a radiator's blast. Extreme cold doesn’t suit it; freezing can mess with its physical structure. Laboratories that regulate temperature help avoid headaches. In cramped corners or makeshift labs, choosing the darkest, calmest cabinet far from heat means fewer chemical surprises.
Original Containers Matter for a Reason
Transferring chemicals into old bottles or mismatched jars sounds convenient, but it’s playing with fire. Labels matter, and chemical-grade containers prevent reactions with unexpected materials. I know a colleague who once used a soda bottle for “temporary” storage. The sorbent lid failed, fumes built up, and that “temporary” fix led to hours of expensive remediation. Using proper containers with clear labeling upfront avoids confusion and medical bills later.
Straightforward Organization Beats Fancy Tricks
Stick to basics: store this compound away from strong acids, strong bases, and anything that oxidizes. That way, accidental spills stay minor annoyances, not full-blown emergencies. Never put unrelated reactive chemicals on the same shelf—one spill can force a building evacuation. Avoid stacking heavy bottles on top of each other. Custodians and first-year students alike appreciate a tidy storage area, and so do insurance companies.
Personal Protection and Clean Environments
Handling chemicals doesn’t just stop at storage. Put on gloves and goggles before handling the bottle. Wipe the outside whenever you use it, so residue doesn’t build up. Cleanliness in storage space prevents tiny accidents from ballooning into health risks. In every lab I’ve worked, the folks who stick to these everyday habits get fewer surprises.
Preparing for the Worst, Hoping for the Best
Routine checks on storage conditions feel tedious but save money and headaches. A log sheet by the cabinet door forces everyone to stay accountable. If your lab’s humidity climbs, invest in better drying equipment. Spills and leaks happen, but if they’re discovered early, lives and research remain unharmed. Plan for problems, stay alert, and treat 1-Propylsulfonate-3-Methylimidazolium Chloride with respect. Secure storage always delivers peace of mind.
Understanding What This Chemical Really Means in Daily Life
1-Propylsulfonate-3-methylimidazolium chloride isn’t a household name. Most people never hear about it unless they step into a lab. Scientists use it as an ionic liquid—basically a salt that stays in a liquid form at room temperature. It finds its place in research, chemical synthesis, and sometimes in green chemistry applications. Whenever chemicals hit the headlines, people rightfully start questioning how safe these substances really are, including what happens if someone spills it, breathes it, or handles it daily.
What Research Shows About Hazards and Toxicity
Most available toxicological data about ionic liquids focus on how they break down, what types of organ systems they affect, and if they have any environmental fallback. Studies on chemicals with similar imidazolium structures suggest that many of them don’t degrade quickly in nature. They can build up over time, especially in water. Some research published in journals such as Environmental Science & Technology found that certain imidazolium ionic liquids show acute toxic effects on aquatic organisms. Some fish and tiny water dwellers didn’t thrive after being exposed. This doesn't always mean every single compound in the class is highly dangerous, but it does raise red flags.
People mistakenly think anything labeled “green chemistry” can’t be toxic. Truth is, just because ionic liquids like this one have low vapor pressures and don’t easily ignite doesn’t make them harmless. Handling 1-propylsulfonate-3-methylimidazolium chloride can irritate the skin and eyes. Those who deal with it directly, often in labs, must wear gloves and eye protection. If someone breathes dust from this salt, they might feel throat or lung irritation. Chronic exposure details stay fuzzy because long-term studies on humans haven’t been published.
What People Like Me Notice in Real-World Use
I’ve had experience working in a research lab, where the approach is always erring on the side of caution. We treat every ionic liquid with respect regardless of the “green” stamp on the bottle. A small spill means prompt clean-up, proper ventilation, and recordkeeping. Nobody assumes there’s zero risk. In labs, waste goes through proper disposal procedures instead of heading down the drain, since the environmental impact hasn’t been mapped fully yet.
I see discussion threads in scientific communities where chemists share experiences using ionic liquids. Some describe mild skin reactions. Others talk about the importance of local exhaust and handling powders gently to avoid airborne exposure. The substance has not been pushed into widespread manufacturing. In this sense, its potential for large-scale environmental release looks low for now. Still, these anecdotes reinforce the role of caution.
Mitigating Risks and Looking Ahead
Chemical producers post clear safety data sheets online for 1-propylsulfonate-3-methylimidazolium chloride, outlining exact first-aid steps, recommended PPE, and procedures in case of accidental release. Institutions encourage training to make sure nobody mishandles the substance unknowingly. Regulations can move slowly but stronger rules about producing, using, and disposing of ionic liquids could cut down risks.
Green chemistry does need better transparency about both knowns and unknowns. For 1-propylsulfonate-3-methylimidazolium chloride, open reporting of toxicity studies, both in labs and real-world scenarios, helps everyone—from chemists to local residents. Chemicals don’t demand panic, just honest communication and a respect for the facts.
Understanding the Chemical Before You Get Started
I’ve spent years working in both academic and industrial labs, handling all sorts of ionic liquids. 1-Propylsulfonate-3-Methylimidazolium Chloride stands out. This compound looks like a regular salt, but don’t be fooled—the properties are far removed from table salt, and how you introduce it to a solution makes a real difference. The sulfonate group brings strong polarity, which helps dissolve in water, yet the organic backbone can stump less experienced chemists who try to take shortcuts.
The Right Way to Start Dissolving
My best results always come from slowly adding the powder to room-temperature distilled water while stirring gently. The compound dissolves much better when you give it the time and space it likes. Rapid pouring leads to lumps sticking together, which I learned the hard way at two in the morning, waiting for a sample to clear. Starting with clean, deionized water helps remove minerals that might interact with the ions and slow you down. If you’ve ever had “ghost” solids swirling at the bottom of a flask, you know the pain this can cause later in your application.
Heat and Patience Go Hand-in-Hand
I’ve seen a lot of folks turn up the temperature as a shortcut. While gentle heating to 40–50 °C definitely speeds things along, excessive heat can trigger decomposition or push the solution past its optimal working conditions. In the early days, my impatience led to off-smelling solutions—not great for analytics or further synthesis. A magnetic stir plate simplifies the process, breaking up the salt’s surface tension and making the mixing even. A simple glass rod works for smaller batches, so there’s no excuse for clumped crystals.
Why Order of Operations Matters
Tossing in other additives or co-solvents before the main ingredient always made my results unpredictable. I learned quickly that solvents with lower polarity, like acetone or ethanol, just make life difficult. Stick to water or a compatible buffer if your downstream work allows. If you need to add buffers or other reactants, always let the imidazolium salt fully dissolve first. There’s wisdom in patience—rushing too many components together leads to uneven solutions that can wreck the precision of whatever you’re building, from catalysts to sensors.
Dealing With Big Batches and Scaling Up
On an industrial scale, agitation mechanics matter. I remember a plant trial where batch size tripled, and suddenly the old methods didn’t cut it. Overhead stirrers with high-torque motors helped distribute heat and ensured every last bit dissolved—no more “hot spots” or crusted residue on vessel walls. For a research setting, it’s less complicated, but don’t ignore the basics. Breaking down lumps by pre-pulverizing the salt with a mortar helps make the process faster and more even when you scale is beyond a gram or two.
Keeping Clean Solutions
Filtration is a step some skip, thinking ionic liquids always dissolve cleanly. That’s not the reality I’ve seen. Microscopic debris or leftover silica from the synthesis can tag along into your solution, causing downstream headaches. A quick vacuum filtration through a fine membrane ensures your work is reproducible. If purity matters in your project—and it really does for most—don’t neglect this step.
Final Thoughts on Good Lab Practice
Taking time to dissolve 1-Propylsulfonate-3-Methylimidazolium Chloride properly builds reliability into your experiments. Every experienced chemist I know has a cautionary tale about skipping steps only to end up repeating the work. Good technique means fewer surprises and better science. A little effort upfront pays off with predictable results and less wasted material.