1-Sulfobutyl-3-Butylimidazolium Chloride: A Comprehensive Commentary
Historical Development
Scientist curiosity about ionic liquids goes back decades, though early examples came out somewhat by chance during work with aluminum chloride salts. Over the years, advances in organic chemistry and electrochemistry made a whole class of room temperature ionic liquids possible, and from that, compounds like 1-Sulfobutyl-3-butylimidazolium chloride started showing up in research reports. At first, these new substances looked interesting because they defied expectations—liquids where most salts acted like rocks. Laboratories tried all sorts of new imidazolium-based formulas, and the sulfoalkyl-modified types drew attention for their mix of solubility, stability, and ionic conductivity that ordinary salts could not touch.
Product Overview
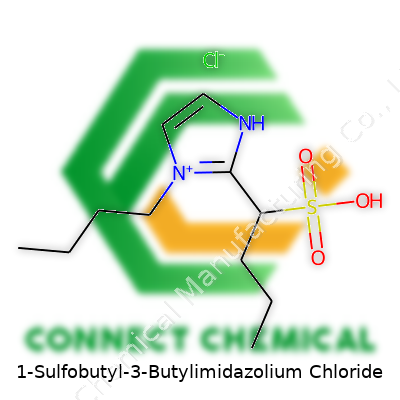
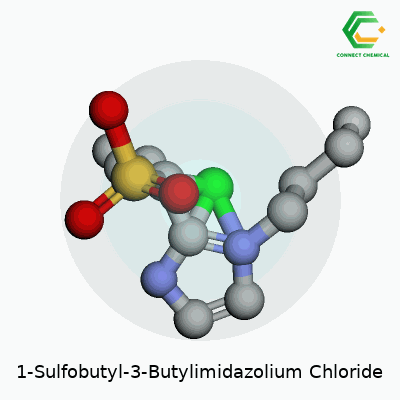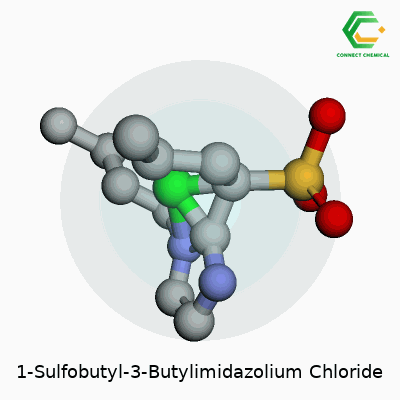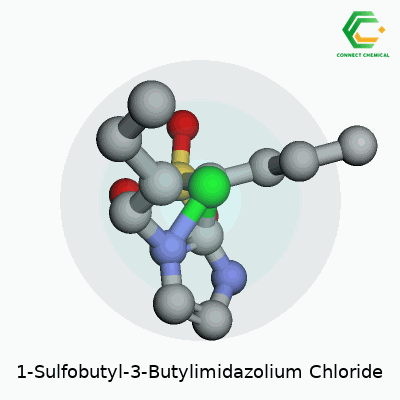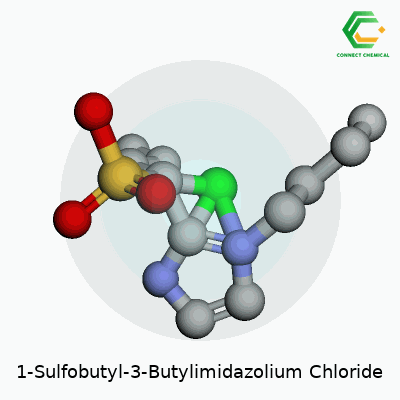
In practical terms, 1-Sulfobutyl-3-butylimidazolium chloride stands out as a liquid salt at room temperature. The structure comes from an imidazolium ring, a butyl chain, and a sulfobutyl group tethered on separate nitrogen atoms, all offset by a chloride anion. This combination means the molecule resists breakdown and deals well with both hydrophilic and hydrophobic environments. Its continued appearance in chemical catalogs comes from demand in labs that work on extraction, catalysis, or novel battery electrolytes. Stability, low vapor pressure, and solid conductivity remain big selling points for development teams.
Physical and Chemical Properties
Most people know right away that this salt pours as a viscous, nearly colorless liquid under typical lab conditions. If you check the melting point, the reading usually falls below 40°C, making it different from older salts that want to crystallize into stubborn blocks. The density hovers just above that of water, pushing about 1.1-1.2 g/cm³. Its ability to dissolve both polar and non-polar materials matches what chemists seek out for greener solvents, especially since the chloride counterion does not mind shifting charges in redox environments. The molecule stands up to common acids and bases and keeps working through repeated heating-cooling cycles, thanks to the strength of the imidazole core and the sulfonate’s electron-withdrawing pull.
Technical Specifications and Labeling
Commercial samples typically come marked with assays above 98%, and reputable suppliers show water content under 0.1%. Storage guides read “keep in airtight containers and out of sunlight,” since slow absorption of ambient moisture does occur if left exposed. Proper chemical names reflect the IUPAC conventions, yet most labs just drop “SBIM-Cl” for ease when scribbling in a notebook. Labels warn against mixing with strongly oxidizing agents or heating above 200°C, since decomposition may toss out toxic fumes. Data sheets usually mention batch-specific residual contamination, at low ppm, from starting halides or intermediately formed organic sulfonates.
Preparation Method
Crafting 1-Sulfobutyl-3-butylimidazolium chloride relies on a two-step method: first, the formation of a sulfobutylimidazole core, then alkylation with an alkyl halide. In a typical small-scale setup, the chemist combines imidazole with 1,4-butane sultone under controlled temperature, usually around 60-70°C. After hours of stirring, 1-sulfobutylimidazole forms, providing a sulfonic acid handle on the side chain. Careful neutralization with sodium hydroxide keeps things under control. In the next move, reaction with 1-chlorobutane kicks off the quaternization, locking in the butyl group and generating the chloride as the anion. Purification by activated charcoal and repeated extractions mop up unreacted starting material, while a final drying step using vacuum yields a clear liquid.
Chemical Reactions and Modifications
Having a sulfonic acid group gives this ionic liquid a head start for all kinds of chemical tailoring. Neutralization turns it into a sulfonate salt, while reactions with different alkyl halides remake the side chain for custom properties. As a solvent, it stands up to strong bases, acids, or transition metal catalysts without falling apart. People working in catalysis look for these abilities, since the liquid does not react with common substrates. Some research uses this class of ionic liquids as both a reaction medium and a phase transfer agent, since it dissolves both organic and inorganic compounds freely. Efforts are already showing that new anion swaps—like pairing the same imidazolium cation with tosylate or hexafluorophosphate—sharpen physical features for battery work or separations.
Synonyms and Product Names
Chemists love shorthand, and this material appears as SBIM-Cl in books or product catalogs. Sometimes the full name reads as 1-(4-sulfobutyl)-3-butylimidazolium chloride. Laboratories have seen alternative spellings, and a few purveyors include SKBIM or SBBIM-Cl. None of these mixes up with traditional quaternary ammonium or phosphonium salts, as the imidazolium backbone gets a recognized spot in modern ionic liquid naming schemes. Product catalogs often place it in the “functionalized ionic liquids” category, since its tailored side chains push the limits of solubility and reactivity well beyond plain alkyl types.
Safety and Operational Standards
Handling requirements line up with most lab-grade ionic liquids. Anyone pouring or transferring needs to wear splash goggles, gloves, and long sleeves since even minor spills cause skin irritation for some workers. Fume hoods turn into necessity when heating above 100°C or mixing with strong acids since byproducts like HCl can form. Disposal guidelines now track local EPA and REACH regulations, calling for solvent recovery or combustion in permitted incinerators because the compound resists simple biodegradation. Fire hazards stay low under normal conditions, but staff never store it with strong oxidizers for safety. Product safety summaries call for immediate clean-up of small spills and reporting of larger leaks, especially in areas lacking good ventilation.
Application Area
Advances in industrial chemistry and green process design have pulled these ionic liquids into focus as alternatives to volatile organic solvents. Extractive metallurgy outfits use SBIM-Cl for selective metal separation, soaking up platinum, palladium, or rare earths from tough ore leachates. In the energy field, battery engineers experiment with it as a part of cutting-edge electrolytes that boost life span and resist flame. Research teams in biocatalysis take advantage of the low toxicity and enzyme-friendly profile, opening doors for organic reactions that run under mild conditions without fouling up sensitive proteins. Wastewater treatment pilots run trials to capture heavy metals from industrial flows, pushing clean-up routines that other solvents simply cannot manage. Out in academia, phase transfer catalysis and supramolecular synthesis groups find this ionic liquid flexible enough to jump between water, oil, and diverse chemical environments depending on project needs.
Research and Development
In recent years, efforts have shifted from basic cataloging to real-world applications that stretch performance and environmental safety. Scientists probe the interactions with metal ions, organometallic complexes, or advanced polymers, looking for ways to combine ionic liquid chemistry with sustainable processing goals. Computational chemists model solvation and conductivity behavior to squeeze every bit of performance out of next-generation electrolytes. New projects chase up recycling of used ionic liquids, cutting the total waste output for sensitive industries. The tweaks on the cation and anion side show tailored viscosity, melting point, and chemical compatibility—critical levers for applications ranging from batteries to pharmaceutical processing.
Toxicity Research
On toxicity, early optimism faded after initial pilot studies. Detailed testing points out that some imidazolium-based ionic liquids possess moderate toxicity toward aquatic life, mainly from the imidazolium ring and byproducts rather than the sulfonic side chain. In vitro work on mammalian cells suggests that extended exposure at high concentrations disrupts cell membranes, though effects stay mild compared to traditional chlorinated solvents. Studies in fish and invertebrates highlight the slow breakdown rate in natural water, raising ongoing concerns for environmental persistence. These results drive the move toward shorter alkyl chains and further functionalization, aiming for ionic liquids that do their jobs without lingering long in the ecosystem.
Future Prospects
Looking forward, the compound’s role in sustainable technology keeps growing. As battery designers embrace ionic liquids for safer, longer-lasting devices, demand for variants like SBIM-Cl follows. Regulatory trends nudge industrial firms towards less volatile and less toxic solvents, a strong case for continued R&D. The quest to separate critical metals and recycle rare earths brings these compounds into hydrometallurgical pilot plants in ways never seen before. Future generations will likely see improved biodegradability, molecular tweaks for even lower toxicity, and further integration with biodegradable or bio-based anions. With wider adoption in green chemistry, material sciences, and waste recovery, this ionic liquid starts to look less like a specialty item and more like a standard fixture in the chemist’s toolkit.
The Reach of a Unique Ionic Liquid
Every year, more researchers and manufacturers move away from old-school solvents. Concerns about toxicity, waste, and risks in handling drive the search for something better. Enter 1-Sulfobutyl-3-Butylimidazolium Chloride—a mouthful, but a real workhorse in labs and production plants. This ionic liquid steps up where water, alcohols, or petroleum-based solvents fall short.
Green Chemistry Gets a New Ally
Chemists love this compound for its stability and low volatility. It won’t go up in a puff of hazardous vapor. During work as a bench chemist, I often found volatile organic solvents tough to manage, both for my health and the environment. Switching to ionic liquids like this one made a noticeable difference. Waste streams became safer, and the air in the lab felt less harsh. In practical terms, this compound gives industries a realistic way to meet environmental rules without wrecking the bottom line.
A Closer Look at Real Applications
Extraction and Separation: Purifying precious metals or separating organic compounds gets much simpler with 1-Sulfobutyl-3-Butylimidazolium Chloride. In battery recycling or mining, companies value its ability to dissolve select substances. Studies published in journals like Green Chemistry support its high selectivity and recyclability. More efficient processes mean less mining waste and better resource use.
Electrochemistry: Modern batteries and capacitors depend on safer, high-performing electrolytes. Here, this ionic liquid brings thermal stability and strong ionic conductivity to the table. Teams working on next-generation lithium-ion batteries use it as a medium for ion transfer. Failures from overheating or short circuits become less likely. Devices last longer, and worries about explosions or fires in consumer electronics drop.
Catalysis: Enzyme and metal catalyst systems run cleaner in these liquids. Synthesis time for fine chemicals drops. Drug makers get purer products with fewer process steps. As someone who’s optimized catalyst systems, I’ve seen how this compound supports high reaction rates and allows easier separation of products from mixtures. Fewer byproducts and less cleanup go a long way in the competitive pharma world.
The Industrial Scale-Up
Scaling up from flask to factory brings fresh headaches. One challenge I ran into early on involved cost—ionic liquids don’t come cheap yet. Still, the fact that 1-Sulfobutyl-3-Butylimidazolium Chloride can be recycled many times offsets some of the expense. Over the last decade, suppliers improved manufacturing routes, leading to higher purity at better prices.
Companies also watch toxicity and biodegradability. Thankfully, compared to classic aromatic solvents, this compound shows much less environmental persistence. A 2022 review in Chemosphere found lower aquatic toxicity and biodegradation rates than halogenated solvents, pointing to a better long-term profile.
Future Directions and Solutions
Industry leaders and scientists should focus on two things: making ionic liquids cheaper, and mapping out their entire environmental journey. Research into production using renewable feedstocks could push costs down further. Life cycle analyses, from cradle to grave, will help uncover hidden impacts and avoid trading one problem for another.
Colleagues in engineering talk about plugging green ionic liquids into existing processes. Done right, 1-Sulfobutyl-3-Butylimidazolium Chloride offers a more sustainable path for energy storage, pharmaceuticals, and rare materials recovery. Putting safer solvents into real-world processes isn’t just good for press releases; it creates practical benefits for workplace safety and environmental stewardship.
Understanding the Building Blocks
Getting familiar with 1-Sulfobutyl-3-Butylimidazolium Chloride, folks will notice two main parts: a modified imidazolium ring, and a chloride anion balancing the charge. The imidazolium ring, known for its stability and electronic properties, forms the backbone of many ionic liquids. In this compound, the ring connects to a butyl chain and a sulfobutyl group, both of which bring their own behaviors to the table.
The Structure Up Close
At the heart, there’s a five-membered imidazole ring. The butyl group attaches at the third nitrogen, offering flexibility and structure. On the other side, the sulfobutyl chain comes off the first nitrogen. This chain features four carbons, with a sulfonate (–SO3-) group at the end. The presence of these groups boosts solubility and ionic mobility, often making the compound a popular pick in advanced chemistry labs.
These charged sections do more than just sit pretty. The big, spread-out cation mixes well in polar and nonpolar situations. Adding the chloride anion gives it balance, so the end formula lands as C11H21N2O3SCl. This diverse mix lets scientists tap the compound for things like electrochemistry, solvent systems, or ion exchange.
Complexities in the Formula
Seeing that formula, it helps to picture the groups as structural players:
- Imidazolium core (C3N2H5)
- Butyl (C4H9) off the third nitrogen
- Sulfobutyl (C4H8SO3-) off the first nitrogen
- Chloride (Cl-) as the counterion
Each of these influences how the molecule interacts. The negatively charged sulfonate group and the chloride anion make it hydrophilic, so it dissolves nicely in water. The butyl group helps the cation slide between polar and nonpolar environments, which opens more doors for use in separation science, sensors, or as a safer route for handling hazardous reactions. My workbench saw decent results swapping out traditional volatile solvents with this salt in extractions, since the compound sticks around and doesn’t add flammable fumes.
Importance and Applications
Looking at the whole picture, the chemical design answers some longstanding lab headaches. Lots of folks expect ionic liquids to come with quirks—thick, hard to handle, or slow to dissolve. 1-Sulfobutyl-3-Butylimidazolium Chloride breaks that cycle with its broad tolerance. Electrochemists use these salts in advanced batteries—they stand up to heat, resist decomposition, and don’t evaporate like old-school solvents. In pharmaceutical development, the sulfobutyl group means strong binding to other molecules, which sometimes pulls tricky actives into solution.
Fact-Based Challenges and Hopes
Handling salts with special structures comes with environmental and safety duties. Many ionic liquids, including this one, show low volatility. This helps cut air pollution, but disposal still takes care. The sulfonate tails can bring persistent bioactivity outdoors, so process engineers focus on reuse or recycling. Better green chemistry starts with tuning side chains—adding or swapping functional groups to keep toxicity low and biodegradation high.
From the hands-on angle, folks value reliable data—melting point, solubility, resistance to breakdown under tough conditions. Standardization moves the work out of the small-scale lab, so industries can design cleaner processes. Repurposing these molecules in new fields—analytical tech, drug design, energy storage—means every structural detail ends up mattering out in the real world.
Getting Beyond Buzzwords in Green Chemistry
1-Sulfobutyl-3-butylimidazolium chloride pops up in discussions about ionic liquids, often tagged as “green” thanks to its reduced volatility and lower flammability compared to old-school solvents. Plenty of industries swapped out harsh chemicals for options like this, hoping to cut back on pollution and hazards in the workplace. But the simple label of “green” doesn’t promise anything about how something behaves after it leaves a lab bench. Talking about real environmental impact means digging up more than just some safety data or technical specs.
What Happens After This Chemical Gets Used?
Understanding the environmental story of this compound starts with a question every chemist and manufacturer must face: Where does it go once the job is done? Some ionic liquids stick around longer in the environment than what feels comfortable. 1-Sulfobutyl-3-butylimidazolium chloride’s structure, with both its bulky imidazolium core and the butyl chains, resists being chopped up by all the microbes you hope will digest it in soil or water. Many peer-reviewed studies highlight how few species of bacteria or fungi seem interested in using these salts as a food source. The result is that these compounds can pile up, especially near large-scale users that don’t recycle or capture waste responsibly.
Comparing Real-World Hazards and Biodegradability
Some research checked the degradation rates under typical aerobic and anaerobic conditions. Results rarely give reason to celebrate—they call this and related compounds “poorly biodegradable.” A study from Environmental Science & Technology detailed limited breakdown even after months. Synthetic chemicals like this might not hunt fish or insects actively, but trouble comes because they disrupt organisms’ membranes and can slow down critical ecosystem functions. Their persistence means low doses add up, affecting aquatic life in ways we’re still mapping out.
Green Chemistry Needs More Than Low Volatility
Deciding if something helps or hurts the planet doesn’t stop at the factory gate. Low volatility cuts down on smog and workplace risks; I worked a summer internship at a plant that shifted away from solvents with high vapor pressure. Our air got cleaner and the headaches faded, but wastewater started grabbing public attention. We learned quickly that environmental claims need to cover the full picture—what goes down the drain matters just as much. Biodegradability often makes the real difference between a cleaner world and new forms of pollution.
Supporting Facts and Solutions
OECD guidelines set specific tests for classifying something as “readily biodegradable.” 1-Sulfobutyl-3-butylimidazolium chloride scores poorly. That’s worrisome given the drive to expand its use, especially in battery tech, chemical synthesis, and extractions. Germany’s Federal Environment Agency flagged several imidazolium-based liquids as substances worth keeping out of open environments unless there’s strong recovery and waste handling in place. Recycling efforts help—closed-loop systems cut emissions—but many small-scale users don’t have this infrastructure. Stronger policies on end-of-life management, thorough hazard testing, and clear guidance will make the biggest dent.
Choosing Chemicals With the Whole Lifecycle in Mind
Talking to folks who work in green chemistry, there’s a growing sense that the job isn’t done once a chemical stops being toxic or flammable. They want products that break down once their work is over. The answer sometimes comes from letting regulators push for safer choices, but real progress also comes from researchers designing new molecules harder for nature to ignore. Lower hazard, higher degradability, and smart stewardship put together the toolkit for tomorrow’s industry—a path that means more than swapping out old hazards for new ones that stick around far too long.
Why It Deserves Attention
Anyone who has worked in a lab setting knows that not all chemicals behave the same way. Some only require standard rules—gloves, goggles, maybe a fume hood. Others, like 1-Sulfobutyl-3-Butylimidazolium Chloride, ask for a closer look. It’s an ionic liquid that finds its way into batteries, catalysis, and even drug delivery systems. That’s no small role in today’s research and tech landscape. It’s sensible to give serious thought to how we keep both people and results safe.
Real-World Storage Habits
Based on years in research labs, the phrase “cool, dry place” gets thrown around a lot, but a few critical points stand out for this compound. Moisture really doesn’t mix well here. The chloride ion will pull in water if given the chance, which can degrade both purity and performance. Sealing the bottle tight after each use will keep problems at bay. If the container forms condensation—if you see droplets inside—it’s time to rethink where you’re placing it. A desiccator or a cabinet with drying agents performs much better than open shelving next to a window.
Sunlight doesn’t help either. Some users have left ionic liquids exposed near windows just for the sake of convenience. Direct light and heat will speed up decomposition and can introduce new impurity signals that show up in your next analysis. Labs report better batch consistency by using opaque storage containers. Some even wrap bottles in foil out of habit. It’s a simple trick that delivers a long shelf life.
Why Gloves Matter
Skin contact with ionic liquids can trigger irritation. Speaking with occupational health staff, the consensus remains clear: double up on nitrile gloves when transferring or weighing this compound. Not only do nitrile gloves resist breakthrough better than latex, but their tight fit means spills won’t sneak past the cuff as easily. Remember, a little slip can turn into persistent skin woes or allergic responses down the road.
Goggles matter, too. In my own lab, I saw someone ignore eye protection on a slow day and end up with a splash injury. Even “mild” chemicals sting and inflame when they hit the eyes. Taking two seconds to grab proper eyewear still beats an emergency rinse at the eyewash station.
What To Avoid
One behavior that trips up even skilled lab workers: using makeshift scoops or spatulas. Those tools may hold on to remnants from other compounds. Cross-contamination derails sensitive work and risks an unintended reaction. Clean, dedicated utensils for handling 1-Sulfobutyl-3-Butylimidazolium Chloride means fewer surprises.
Waste management plays a role, too. Dumping this kind of residue down the drain violates environmental rules and causes real harm to water systems. Seal any leftover or spilled material in a labeled hazardous waste bag. Disposal services have protocols that protect both people and planet. If there’s any doubt, contact institutional safety staff rather than guessing.
Better Safety Through Knowledge
Storage and handling protocols aren’t just regulatory boxes to tick—they reflect the sum of accidents, near-misses, and learned best practices across the research world. Check the latest data sheets for handling 1-Sulfobutyl-3-Butylimidazolium Chloride. Visit those annual safety trainings with an open mind. Share close calls with colleagues so the next person learns before luck runs out. True experience isn’t just about the science, but caring enough to keep yourself—and those working with you—healthy and safe.
Pursuing the Right Purity, Without Nonsense
Purity always draws curiosity, especially from companies or researchers preparing to invest in bulk raw materials. Let’s be honest—no one wants a product with question marks all over its label. The purity number, often stamped at 95%, 99%, or even above, quickly separates top-tier suppliers from those just looking to make a fast sale. In pharmaceutical labs I’ve worked with, the expectation is set: contaminants can’t slip in the backdoor. At the end of the day, humans receive these compounds. Even a trace of unwanted byproduct can threaten an entire batch, waste months of work, or in some industries, actually put end users at risk.
Beyond health, shoddy purity ravages budgets. No manager wants to pay for 100 kilograms expecting high-grade material, only to send it back or watch production stall because the initial batch didn’t meet spec. That’s a mistake even a rookie procurement team remembers.
Why Numbers Matter More Than Fluff
Lab reports offer more than a single purity number. Take a real example: a chemical advertised at 99.5%. The best vendors will share a certificate of analysis attached to each shipment. Those sheets show breakdowns. Moisture, metals, potential byproducts: every line tells a story. It’s worth checking if your vendor hides behind just one number or puts the whole data set in plain sight.
It’s not always about hitting 100%. Some applications, like advanced electronics or sensitive medical formulations, demand ultra-pure stocks. Food and beverage sectors might accept 98%—but only if the impurities pose no threat to flavor or safety. Ask tough questions. If a supplier can’t show their process purifies ruthlessly or struggles to answer how much lead or arsenic sits below threshold, their product deserves scrutiny.
Quantities at Scale: No Smoke and Mirrors
One-off research samples? Most can find a few grams or a kilo online. Scaling up to industrial use—hundreds, thousands of kilograms—presents another beast. From experience, delivery timelines get stretched if the supplier tries to drum up the volume last minute. Reputable distributors keep supply data honest. They’ll either maintain regular warehouse stock or establish lead times upfront, with contract guarantees attached.
Bulk buyers don’t just want a quote; they want logistical confidence. Questions worth asking include: Does the company keep a rolling batch record? Are backups in place in case a shipment falters? How do they pack large lots—in sealed drums, lined sacks, custom containers?
Experienced buyers know to check export documentation, hazardous goods rules, and how sensitive material ships under varying temperatures or customs controls. Smaller companies, new to the game, can get tripped up if they lack clear answers about temperature or pressure shocks and non-compliant packaging.
Cut Down the Guesswork
In my years working alongside procurement teams, the ones that struggled most jumped at the first low bid, ignored the finer print, or never pushed the supplier for proof. There’s no shortcut: get the purity specs in black and white, ask to see storage and shipping protocols, and request batch consistency records.
If enough buyers ask these tough questions, the quality bar rises for everyone. The right purity and volumes shouldn’t feel elusive. Reliable documentation, tested processes, and transparency should be the bare minimum, not the exception.