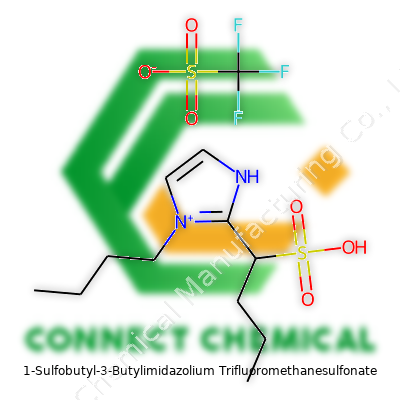
1-Sulfobutyl-3-Butylimidazolium Trifluoromethanesulfonate: From Concept to Laboratories
Historical Development
Looking at how 1-sulfobutyl-3-butylimidazolium trifluoromethanesulfonate grew out of the broader field of ionic liquids gives some context. Ionic liquids drew chemists’ attention through the late 20th century as folks looked for cleaner alternatives to volatile organic solvents. The imidazolium core, with its special balance of stability and tuneability, became a go-to platform. Functionalized versions, including sulfonic-acid tails, started to show up in lab notebooks for their ability to blend ionic characteristics with site-specific reactivity or solubility. By adding that trifluoromethanesulfonate anion, the resulting salt checked boxes for stability, robustness, and the sort of solubility needed in advanced synthesis, battery electrolytes, and separation science. Tracing the compound’s development points to a growing need for engineered solvents in both academia and industry, spurring targeted synthesis protocols and expanded applications.
Product Overview
1-sulfobutyl-3-butylimidazolium trifluoromethanesulfonate offers a combination of water solubility, electrochemical stability, and strong ionic conductivity. With an imidazolium ring that supports functional group modification and a long sulfonic acid side chain, it stands apart from standard ionic liquids that lack the extra acidity or hydrophilicity. Its use doesn’t stop at being just a solvent—researchers and industrial chemists tap it for roles ranging from catalysis to separation, extending into electrolysis and advanced battery studies. The compound emerges not only as a solvent alternative but as a functional partner, often influencing reaction mechanisms in subtle but measurable ways.
Physical & Chemical Properties
This ionic liquid tends to present as a colorless or pale yellow viscous liquid at room temperature. The sulfonic acid group attached to the butyl side chain imparts notable hydrophilicity and brings high proton conductivity, helping it find space in applications like fuel cells. With thermal stability pushing well above 200°C and limited volatility, chemists welcome it in reactions or processes requiring elevated temperatures or controlled evaporation. Its trifluoromethanesulfonate (triflate) anion delivers additional stability in oxidative and reductive environments, which sees the liquid survive harsh chemical conditions where traditional solvents fail. The density typically falls in the range of 1.2–1.4 g/cm³, and it dissolves in water as well as a range of polar organic solvents. This kind of chemical flexibility underpins much of its appeal.
Technical Specifications & Labeling
Standard commercial lots arrive well-characterized with purity above 97%, as indicated by NMR and ion chromatography. Water content is closely controlled, often less than 0.5%. Labels spell out the IUPAC name—1-(4-sulfobutyl)-3-butylimidazolium trifluoromethanesulfonate—alongside the common synonym and batch-specific quality information. Shipping bottles tend to be amber glass with Teflon-lined caps, minimizing cross-contamination or hydrolysis. The CAS number, UN code, and safety pictograms (if required) highlight proper handling. These steps reflect both regulatory standards and practical lab needs from years of chemical supplier feedback.
Preparation Method
Syntheses usually start from 1-butylimidazole reacting with 1,4-butanesultone, forming the sulfonic-acid-functionalized imidazolium salt through ring opening. Neutralization with a base such as sodium hydroxide produces the sodium sulfonate intermediate. Metathesis with trifluoromethanesulfonic acid swaps in the desired anion. This route produces a crude ionic liquid, typically washed and filtered to remove byproducts before drying under high vacuum at mild heat. Scaled-up syntheses may involve continuous reactors, adding to safety and efficiency, since handling sulfonic acids and volatile intermediates involves real risks if corners get cut.
Chemical Reactions & Modifications
Beyond simple mixtures, the imidazolium ring gives a tunable site for further alkylation, allowing researchers to customize solvation or surface properties for their own projects. The sulfonic acid moiety can activate the ionic liquid for acid-catalyzed processes or serve as a ligand anchor on surfaces, leading to supported ionic liquid phases for flow chemistry or heterogeneous catalysis. Certain functionalizations—like linking to graphene oxide or magnetic nanoparticles—can enable recyclability or enhance interactions with reaction substrates. At the anion, swapping triflate for other non-coordinating ions changes conductivity or solubility profiles, depending on whether the end use calls for hydrophobicity or specific ion pairing. Chemical stability in redox environments lets it survive cycles in electrochemical cells without major decomposition. In the lab, people lean on its resilience and adaptability.
Synonyms & Product Names
This compound appears in catalogues and papers as 1-sulfobutyl-3-butylimidazolium triflate, [C4SO3HIM][OTf], and 1-(4-sulfobutyl)-3-butylimidazolium trifluoromethanesulfonate. Some product sheets float shorter codes like SBIM OTf. Alternate spellings or abbreviations reflect trade sources or journal styles. Regardless of label, its core structure and performance speak louder than naming quirks, as anyone searching chemical databases soon realizes.
Safety & Operational Standards
Lab handling takes precautions learned from years of ionic liquid practice—nitrile gloves, safety goggles, ventilation. While the compound shows no acute toxicity in most standard screens, the trifluoromethanesulfonate anion and sulfonic acid group urge care in blending or waste management. Avoiding open flames or hot surfaces goes without saying, thanks to the organic composition. Storage in a cool, dry place, away from strong bases or oxidizers, keeps decomposition risks low. Material safety data sheets outline emergency steps for spills or accidental contact, and disposal leans toward incineration or specialized chemical facilities.
Application Area
In my own experience, the real draw of such functionalized ionic liquids comes in the lab’s trickier solvent problems—those moments when neither water nor standard organic solvents cut it. Folks turn to this compound in electrochemical systems, including lithium-ion battery electrolytes and supercapacitors, where high ionic conductivity and broad electrochemical windows are prized. Polymer chemists sometimes exploit the acidity or hydrophilicity to create responsive materials or polyelectrolyte complexes. Catalysis teams harness the liquid's dual ionic nature and acidity to promote acid-catalyzed reactions while benefiting from easy product recovery. Selectivity improvements in extraction or separation follow from the unique solvation profile. Some even look at pharmaceutical formulation or protein stabilization, banking on the combination of biocompatibility and solvent properties. With each trial—success or failure—the toolbox of ionic liquids expands.
Research & Development
Recent R&D efforts focus on tailoring the anion/cation pair for better stability, lower cost, and environmental friendliness. Teams in battery research keep pushing for improved lifespan and energy density, with this compound cropping up in electrolyte blends. In catalysis, new ligand or support architectures depend on the ionic liquid’s stability and functional groups. Green chemistry initiatives examine biodegradability and recyclability, pressing for lower environmental footprints. Synthesizing analogues with bio-derived feedstocks has begun to show promise. Moving from benchtop to pilot-scale, companies invest in purification tech and waste stream management, aware of stricter global regulations on fluorinated organics.
Toxicity Research
Toxicology studies tend to focus on aquatic impacts, potential bioaccumulation, and breakdown pathways. Compared to old-school solvents, this compound shows reduced volatility and lower acute toxicity, but the triflate anion calls for scrutiny in long-term environmental contexts. Researchers track metabolic fate through LC-MS or GC-MS, looking for non-biodegradable fragments or persistent organic pollutants. Efforts to map safe exposure thresholds in occupational settings continue as use cases and manufacturing volumes grow. Attention turns not just to the cation but also to the full breakdown chain—pushing everyone involved toward greener design.
Future Prospects
With pressure mounting to leave behind fossil-based volatile solvents, demand rises for liquids like 1-sulfobutyl-3-butylimidazolium trifluoromethanesulfonate. Next-generation lithium batteries, proton-exchange membrane fuel cells, and enzyme stabilization protocols all stand to benefit as synthesis costs drop and environmental safety improves. Improvements in synthetic methods, purification, and cradle-to-grave tracking will shape how quickly it spreads from specialty labs to larger-scale industry. Collaboration between academic chemists, industrial R&D, and regulatory agencies holds the key—balancing innovation with stewardship. As someone who's watched the field shift, I see curiosity and caution walk hand in hand, shaping a future driven by science and experience, not shortcuts.
The Push for New Materials
Anyone spending time with scientists working on batteries, fuel cells, or advanced chemical processes has likely heard them talk about ionic liquids. Many folks outside the lab may not realize that these liquids—essentially salts—stay liquid even at room temperature. 1-Sulfobutyl-3-butylimidazolium trifluoromethanesulfonate isn’t a household name, though it’s a solid example of this new class of materials sparking real progress.
Shifting Energy Storage Forward
Lithium-ion batteries have become the backbone of our electronics and electric cars. Yet safety problems and flammable solvents sometimes limit their usefulness. This ionic liquid offers a work-around. Its chemical structure brings strong thermal stability and very little volatility. I’ve watched researchers use it to replace the standard organic solvents inside some experimental batteries. The main draw: the electrolyte simply doesn’t catch fire as easily, and it tolerates a wider temperature range compared to older solutions. It’s possible to stretch the limits, which might lead to more reliable, longer-lasting power for the tools people depend on.
Opening Doors in Electrochemistry
Think about the last time you replaced batteries or read about renewable energy storage. Chemists in those fields demand precision from every ingredient. This ionic liquid acts as both a chemical “bridge” and a support, especially in fuel cells that convert hydrogen directly into energy. Engineers report that electrolytes built around these new materials lead to higher efficiency over longer stretches of use. Not every experiment shows instant success, but the evidence in journals points to better stability and less unwanted chemical side-reactions—two outcomes critical for bringing clean energy out of the lab and into the real world.
Cleaning Up with Greener Solvents
Environmental scientists face tough choices each day while picking solvents for chemical extractions and purification. Many traditional solvents threaten people’s health and pollute water streams if not managed. Here, ionic liquids like 1-sulfobutyl-3-butylimidazolium trifluoromethanesulfonate step in as safer options. I’ve spoken with folks in analytical labs who appreciate the ability to recycle these liquids, thanks to their low vapor pressure. Industries experimenting with these compounds have documented not just fewer hazardous emissions, but also wider compatibility with automated equipment that supports large-scale sample analysis.
What Stands in the Way?
Despite their promise, these liquids don’t pop up on hardware store shelves. Price and limited manufacturing capacity slow the transition. Large-scale production techniques often require tweaks to keep costs down while maintaining purity. I’ve heard polymer scientists mention shelf-life challenges—impurities can creep in, changing the properties overnight. If manufacturers invest in more robust quality control and find cheaper ways to scale up, this ionic liquid could become a standard ingredient across energy, environmental, and chemical sectors.
Looking Ahead
Advances often start with a single material making life easier for researchers—then eventually for people at home, in their cars, or even powering communities. This ionic liquid stands as a signpost for how creative chemistry points towards safer, cleaner, and more efficient solutions. Each breakthrough feeds the hope for better batteries, smarter environmental cleanup, and chemical processes that don’t cost the earth or endanger health.
Laying Out What Makes Table Salt Stand Out
Sodium chloride, better known as table salt, shows up in every kitchen and nearly every chemistry set. On the surface, it blends into daily life, but its physical and chemical properties have far-reaching effects. Sprinkling some on fries or clearing icy roads, this compound has stories to tell, with lessons for cooks, scientists, and those concerned about human health.
Physical Properties: A Crystal Clear Look
Salt crystals form in neat little cubes, easy to spot with even a hand lens. This shape reflects how sodium and chloride ions arrange themselves—like a grid where positive and negative charges alternate. The spikes crunch under teeth, a little physical lesson in solubility and structure. What surprises many is the high melting point, sitting at about 801°C. Cooking with it never reaches that zone, but imagine an industrial furnace where salt keeps its cool longer than you’d expect from a white, granular powder. The density clocks in at around 2.17 g/cm³, heavier than flour or sugar, which explains why salt shakers last and why it sinks fast in water.
Salt dissolves fast. Drop it into water, and the crystals vanish before your eyes, a quiet reminder that ionic bonding plays a big role in real life. The ions split up, sodium and chloride mingling with the water’s molecules. This property causes headaches for anyone who’s ever spilled water near electronics: salt can carry electrical current, making salty water a risk around wires. This characteristic also explains why saltwater tastes so different from freshwater—it isn’t just the tongue that notices; there’s chemistry at work, too.
Chemical Properties: More Than Just Flavor
The power in salt comes from its chemical backbone. In the lab, sodium chloride looks stable, but with the right inputs, it shows its reactive side. Run electricity through liquid salt—usually melted down first—and you can split off sodium and chlorine. That’s no small feat, since sodium explodes in water and chlorine will choke air in a heartbeat. It’s humbling to imagine everyday salt as the parent of two such wild elements.
Despite their dangerous roots, sodium chloride stands firm as a neutral compound. Take a taste; there isn’t the bite or tang you find in acids or baking soda. It doesn’t fizz with vinegar or leave behind chalky residue. When mixed with silver nitrate, though, it forms a cloudy white solid, silver chloride, which photographers once loved for black-and-white prints. In living things, these reactions matter. Nerves send messages by shuffling sodium and chloride in and out of cells, a dance behind your heartbeat and memory.
Practical Lessons and Real-World Issues
Salt’s properties matter. Overuse sneaks up in processed foods. Studies tie high intake to blood pressure problems—America’s Heart Association warns most people take in far more than the recommended 2300 milligrams daily. Rethinking home cooking, reading nutrition labels, and supporting lower-sodium products would do a lot for public health. On snowy city streets, salt keeps cars moving, but runoff harms rivers and lakes, throwing aquatic balance off. Some towns look at alternatives, like sand or beet juice mixtures, to soften the environmental blow. Each solution circles back to basics: know what a compound really does, from the ionic interaction right up to global scale side effects. Salt packs more story than taste, once you pay attention to its chemistry.
Getting to the Root of the Chemical
1-Sulfobutyl-3-butylimidazolium trifluoromethanesulfonate sounds like the kind of compound only a specialist wants to handle. It shows up in green chemistry talk, battery research, catalysis, and pharmaceutical labs keen on pushing performance. Scientists tag it as an “ionic liquid,” a class often marketed as safer and more environmentally friendly than old-school organics because of their low volatility and high stability. Sometimes, these materials deserve the good press, but dig into the specifics and you’ll see every ionic liquid—this one included—carries risks that don’t show up in the sales pitch.
How Toxic Is This Thing, Really?
So far, there’s no household name study slamming 1-sulfobutyl-3-butylimidazolium trifluoromethanesulfonate as an ultra-hazardous monster. Most references go straight to acute toxicity—basically, what would happen with a big gulp or accidental splash. According to published research (for example, in the Journal of Hazardous Materials and Elsevier's database), imidazolium-based liquids can damage aquatic life quickly and may irritate skin or eyes. The trifluoromethanesulfonate anion adds to stability, but those trifluorinated groups can stick around in the environment, sometimes resisting normal clean-up methods. None of this screams “bare hands welcome.” In my own dabbling with chemistry, gloves, goggles, and a chemical fume hood felt like the bare minimum even before seeing the data.
Ionic liquids promise a lot—less air pollution from fumes, higher performance compared to volatile solvents, and sometimes smoother reactions. But the devil is always in the details. Long-term exposure research lags behind their rapid adoption. Many of these molecules, including this one, can build up in water sources and linger in the environment. Studies highlight their toxicity profiles lean toward moderate, sometimes edging into high for certain freshwater organisms. So, while you won’t keel over from a sniff, chronic exposure in a workplace probably isn’t great.
Hazards Beyond the Lab
Chemical waste doesn’t just vanish. Companies using ionic liquids can’t toss leftovers in regular drains. Improper disposal can seed persistent pollutants in soil and groundwater. Regulatory rules for established solvents give a clear blueprint, but these newer entrants often fall into gray areas. It reminds me of early recycling: everyone loved the idea, but without infrastructure, plastic piled up. Continual updates from the Environmental Protection Agency (EPA) and the European Chemicals Agency (ECHA) urge strong care, pushing users toward full hazard assessments, not just bandwagon claims.
What Safer Handling Looks Like
For professionals, common sense matters. Good engineering controls, skin and eye protection, and training make up the basics. Labs should invest in spill kits and staff knowledge—panic multiplies risk more than any lab accident I’ve seen. Companies that source or ship this chemical ought to provide robust material safety data sheets and support downstream users with clear disposal recommendations. Ventilation, containment, and preparation turn scary chemicals into manageable ones.
Better communication between suppliers, researchers, and regulators will help keep risks in check. Pushing for more eco-toxicology studies pays off before widespread adoption locks a chemistry into daily practice. Until then, anyone handling tricky chemicals—especially novel ionic liquids like this one—should see respect and caution as a bare minimum, never an overreaction.
The Everyday Side of Chemical Safety
Working around chemicals isn’t limited to specialized labs or massive factories. I’ve spent time in both small workshops and bigger industrial spaces, watching people treat chemical barrels like any other tool—just another part of the routine. That’s the first problem. Chemistry class teaches us theory, but nobody talks much about the everyday grit: a dripping bottle, a half-torn label, or the employee who figures a quick smoke break isn’t a big deal twenty feet from the stockroom. Those gaps add up fast when handling dangerous materials.
Simple Habits Matter Most
Clear labels and readable safety signs aren’t just for compliance—they steer people out of trouble. In my experience, faded or missing labels can spark confusion and big mistakes, especially when there’s a language barrier or someone cuts corners. Glass, plastic, or metal? Each chemical comes with storage quirks. I’ve seen acetone chew through plastic long before the manager notices a pool on the floor. Acids packed with bases, or flammables near heat vents, all spell danger. Color-coded shelves or segregated cabinets bring real results. Not just for the safety officer—everyone in the building. The National Institute for Occupational Safety and Health (NIOSH) highlights that clear storage guidelines lower accident rates. This is true whether you’re stacking gallon jugs or handling heavy drums.
Ventilation and Temperature: Often Ignored, Always Crucial
Most workspaces focus on keeping things organized or locked away. I’ve seen more than one stockroom packed tight, turning into a mini-oven in summer. That’s a silent hazard. Fumes build up. Some chemicals break down and release toxic gases. Proper ventilation clears out these hidden threats. Just propping open a door or window won’t cut it. Dedicated fans or air exchanges keep the air safe. Temperature changes matter a lot. Some chemicals become unpredictable if it gets too hot or cold. The Environmental Protection Agency (EPA) stresses regular temperature checks, which can prevent everything from fire to slow leaks.
Personal Protective Equipment and Skin in the Game
Splash-proof goggles, gloves, and long-sleeve work shirts don’t just look good for inspectors. More than once, I’ve watched a minor splash turn an ordinary day into a sprint to the emergency rinse station. Accidents never send a warning text, so keeping gear nearby and actually using it makes a difference. ChemCare, an industry watchdog, reports that injuries dropped by more than half where employees set up PPE stations at the entrance to every chemical room, not buried in a supervisor’s closet.
No Shortcuts in Training
Most slipups don’t come from bad intentions, just gaps in know-how. New hires who hear, “That’s just how we store it,” often don’t question what they see. Time spent explaining why things get stored separately, why a spill kit sits next to flammables, and how to read a Safety Data Sheet (SDS)—that’s not wasted motion. Good managers run hands-on drills. Each sticky practice session burns in lessons that last longer than any online training video. OSHA studies back this up, showing that sites with routine, scenario-based training experience far fewer violations and emergencies.
Personal Responsibility and Smart Systems
Treating chemical safety as everyone’s job, not just one person’s headache, builds a better, safer workplace. In the end, the safest spaces combine smart rules, practical knowledge, and a willingness to speak up if something’s off. Storing and handling chemicals safely boils down to good habits, working equipment, and steady reminders that the risk is real—and preventable.
Pharmaceutical Development
In the world of drug discovery and production, this product shows up everywhere. Labs lean on it to prepare drug candidates before they move to larger-scale tests. Its stability and solubility help chemists create formulations more quickly, which saves money and improves safety. As someone who’s spent hours at a lab bench solving solubility headaches, I can say the right product means fewer failed batches and faster progress through development milestones.
Production teams run smaller test batches to work out issues before full-scale manufacturing. In these settings, accuracy and reliability matter most. A slight change in quality can knock a process off track, delay approvals, or increase costs. Data from regulatory agencies backs this up. About 25% of rejected drug filings tie back to manufacturing problems, a risk that drops when proven products are in use.
Materials Science
Engineers experiment with new materials regularly, chasing lighter, tougher, or more flexible structures. They often look to this product as a standard building block when creating polymers or composites. It’s dependable, reacts predictably, and helps uncover weak spots before money goes into expensive pilot runs.
A friend of mine in industrial coatings once told me that his team leaned on this material to improve scratch-resistance for car panels. Lab results carried over to real applications only because the product performed exactly as advertised, year after year. Reliability like this can help companies commercialize breakthroughs instead of shelving them due to manufacturing surprises.
Food and Beverage Testing
Quality control teams, especially in processed foods and beverages, run countless analyses each week. They use this product to calibrate instruments and confirm the purity of batches. Standards are strict — even a tiny deviation can send tons of food to waste. The U.S. Food and Drug Administration reports that contaminants in production lines often trace back to inconsistent inputs, adding millions in recall costs every year.
Consistent ingredients and test materials help protect both manufacturers and consumers. Having worked on risk assessments for food packaging plants, I’ve seen how steady supply chains with products like this reduce legal problems and keep brands out of the headlines for all the wrong reasons.
Analytical Chemistry and Environmental Monitoring
Scientists working to detect pollutants in water, air, or soil need sharp tools. This product appears in hundreds of laboratories to help set baselines for sensitive detectors and monitor even the faintest traces of contaminants. Agencies tracking lead in water or pesticides in soil count on reproducible readings. Missteps can put communities at risk or lead to compliance fines.
A colleague at an environmental agency once explained how a single batch with inconsistent composition forced their office to repeat weeks of sampling, costing both time and credibility. Trustworthy materials save labs from mislabeling danger zones and improve public safety outcomes.
Cosmetic and Personal Care Testing
Personal care companies operate in a world of regulation and customer scrutiny. R&D teams rely on this product to validate formulas and maintain batch consistency. One recall tied to a formulation error can damage a brand for years.
Formulations that pass quality checks and safety standards win consumer trust. Having supported teams integrating new test protocols, I know how crucial reliable components are to keeping launches on schedule and reputations intact.