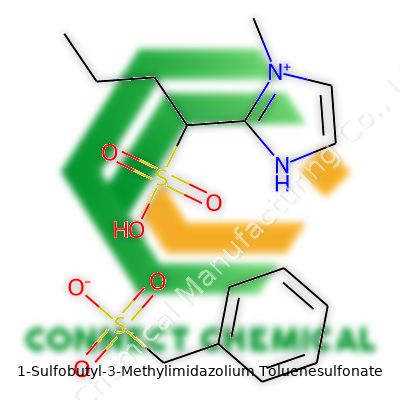
1-Sulfobutyl-3-Methylimidazolium Toluenesulfonate: A Deep Dive
Historical Development
The emergence of ionic liquids like 1-sulfobutyl-3-methylimidazolium toluenesulfonate marks a turning point in advancing green chemistry. Earlier, organic synthesis often relied on volatile organic solvents, which created health and environmental headaches in labs and factories. With the imidazolium family gaining interest in the late twentieth century, scientists were looking for salt-like solvents that dodged flammability and evaporation issues. By adding functional groups—think sulfonate and methyl—they figured out how to tune polarity, solubility, and thermal stability. The pairing of a sulfonated alkyl chain with a common aromatic sulfonate anion arrived as a response to the need for stable, easy-to-handle, and designable liquids. Major breakthroughs came from European research groups, who published papers showing improved yields for catalytic reactions and exciting solubility for biopolymers. Since then, this compound found its way from lab benches to pilot plants, showing that new chemistry follows real-world demands for safety, scalability, and sustainability.
Product Overview
In day-to-day work, 1-sulfobutyl-3-methylimidazolium toluenesulfonate stands out as a clear to faintly yellow, viscous liquid with negligible vapor pressure. As far as ionic liquids go, it combines a robust cation for chemical resilience and the toluenesulfonate anion, which resists oxidation. It shows high thermal stability, clever miscibility with both organic and aqueous phases, and a knack for dissolving a wild range of solutes. This mix of properties gives it a role not just as a solvent, but also as a reaction medium, electrolyte, phase transfer catalyst, and more. Commercial suppliers often target pharmaceutical and materials science firms, thanks to their growing interest in clean, high-value manufacturing.
Physical & Chemical Properties
You can hold this compound up to tough standards. Its melting point usually falls below room temperature, meaning it remains in the liquid state across seasons without fuss. At standard pressure, it doesn’t boil as traditional solvents do; rather, it starts to break down beyond 350°C. Density comes in higher than water, typically above 1.1 g/mL. What jumps out is the high ionic conductivity and strong ability to dissolve both polar and nonpolar molecules. Chemical resistance covers acids, bases, and common oxidants. Because both ions can tolerate heat and don't react with oxygen, labs don’t worry about fire or fume hoods. Its viscosity feels thick, which can slow mixing but boosts selectivity in chemical processes. It absorbs some moisture from the air but doesn’t degrade easily, making it practical in places without heavy-duty climate controls.
Technical Specifications & Labeling
Manufacturers highlight purity, generally topping 98% by weight, with water content kept below 0.5% to ensure high-quality results in sensitive syntheses. Bottles carry batch numbers, production dates, and expiration advice to track material history. Chemical labels detail both the full systematic name and the formula, C14H22N2O5S2, along with hazard notifications focused on skin and eye irritation. Technical data sheets call out decomposition temperatures, storage instructions (tightly sealed, at room temperature), and recommended handling tools—gloves, protective eyewear, chemical-resistant coats—to support safe workplace standards. Transport documents code it under non-dangerous goods, but facilities train staff as a precaution, as with any unfamiliar chemical.
Preparation Method
Synthesis begins with 1-methylimidazole and a haloalkane (like 1,4-butanesultone) in an inert solvent, which forms the sulfonated imidazolium core through ring opening and quaternization. The intermediate then reacts with sodium toluenesulfonate in water or acetone. After heating and stirring, the mixture separates, and the ionic liquid washes away small molecule salts and solvent residues. Vacuum drying under gentle heat removes trace moisture. Every chemist knows the temptation to speed through drying steps, but skipping thorough washing and drying puts downstream experiments at risk. Recrystallization or column chromatography isn't needed given the inherently high purity of the target product and its unique physical properties.
Chemical Reactions & Modifications
Both the cation and anion show unusual stability, but careful chemists test for side reactions before applying them to ambitious syntheses. The imidazolium ring resists nucleophilic attack or acid-catalyzed rearrangements, while the sulfonate chain can anchor catalysts or host transition metals for catalysis studies. As an ionic medium, it holds up under redox cycling, which attracts electrochemistry teams designing safer batteries or fuel cells. Some researchers have attached fluorescent tags or long alkyl chains at the nitrogen site. These tweaks broaden performance in extraction, separation, or enzyme stabilization studies. Each new substitution leads to another round of safety and efficacy evaluation, where even tiny changes alter solvent properties and lab handling.
Synonyms & Product Names
Across the literature and commercial channels, the compound appears with names like 1-sulfobutyl-3-methylimidazolium tosylate, SBMI-Ts, and even more technical terms. Chemists double-check that vendors and journals mean the same product, since small spelling tweaks shift the balance of ions. CAS numbers and structural diagrams cut through the noise, reducing shipment errors or mix-ups in cross-citation.
Safety & Operational Standards
Working with this ionic liquid demands respect for basic lab rules. Though less hazardous than halogenated solvents, it can still irritate the skin, eyes, or lungs. Standard operating procedures include prompt cleanup of spills, use of fume extraction during weighing, and placement of containers away from heat. Safety data sheets recommend nitrile gloves, chemical splash goggles, and closed footwear. In accidents, quick rinsing with water controls most exposures. Material disposal follows local and national rules for organic waste, keeping ionic residues out of municipal water. Chronic toxicity data remains limited, so researchers educate newcomers on careful transfer and labeling—cutting down on confusion during late-night experiments. Since mishandling any specialty solvent undermines results and user health, regular training updates and dry-runs help spot problems before they escalate.
Application Area
This compound sees natural fit for tasks where both performance and safety count. Labs use it to solubilize tough polymers or catalyze organic reactions where traditional solvents fail. Electrochemistry projects benefit from its wide electrochemical window and low volatility, making stable batteries or capacitors more realistic. In pharmaceuticals, it shines during separation of active compounds, especially when you want to sidestep residual solvents in the final product. Enzyme-based manufacturing lines report enhanced biocatalyst activity, given the ionic matrix’s ability to stabilize protein shapes. Environmental teams run extractions with this ionic liquid, cutting out toxic extraction agents and streamlining recycling. Scalability into pilot plants shows up in academic papers and trade journals, suggesting growing comfort beyond academia.
Research & Development
Universities and corporate labs keep exploring how tweaks in the molecular structure impact practical results—from electrical conductivity for next-gen devices to selectivity in industrial synthesis. Big grants fund projects on incorporating these liquids in carbon capture, wearable electronics, and recyclable plastics. Some studies track how new derivatives hold promise for greener processes or smart materials that react to temperature or light. As the research field matures, patent filings outpace journal articles. Open-source databases now catalogue physical data and successful reaction formats, making it easier for new players to catch up without months spent on trial and error. Collaborations across university, government, and industry settings speed progress, while international standards emerge on labeling and batch testing.
Toxicity Research
As ionic liquids like 1-sulfobutyl-3-methylimidazolium toluenesulfonate become more common in industry, labs examine health and environmental effects. Acute toxicity in animal models stays low, but chronic exposure raises questions over bioaccumulation, particularly in aquatic systems. Early ecotoxicity studies suggest low mobility through soil and water, thanks to the stable ionic pairing. Still, responsible manufacturers use this data to tweak disposal instructions and design recovery systems before waste ever hits the drain. The absence of widespread incidents keeps the material out of the regulatory hot seat, yet ongoing monitoring by independent labs ensures rapid response if new hazards develop. Calls grow for life-cycle assessment and data sharing to close the knowledge gap and prevent downstream problems.
Future Prospects
Looking ahead, 1-sulfobutyl-3-methylimidazolium toluenesulfonate appears in forecasts for cleaner chemical plants, safer battery materials, and biodegradable plastics. As new industries look for solvents that balance performance, safety, and environmental profile, future versions may include built-in markers for easier cleanup or enhanced affinity for new classes of catalysts. With the world shifting toward renewable energy and sustainable manufacturing, these ionic liquids look ready to play a role in making both the products and the processes cleaner and tougher. Researchers and engineers focusing on finding the right balance between innovation and caution push the boundaries, keeping a close eye on long-term impacts while squeezing out better results from every drop.
Unlocking the Power of Unfamiliar Chemistry
Chemicals with complex names, like 1-Sulfobutyl-3-Methylimidazolium Toluenesulfonate, rarely get attention unless they spark some controversy. Behind lab doors, though, compounds like this quietly shape modern science and industry. This specific substance lands in a category called ionic liquids. Unlike typical salts, they don’t crystallize into powders. Instead, they stay liquid even at room temperature. That single difference opens up a world of possibilities.
Behind the Scenes in Labs and Factories
Years ago, while working in an analytical lab, I learned just how many old approaches suffer from slow reactions or tough separations. Engineers kept getting frustrated with evaporating solvents or harsh conditions that wasted energy and resources. Someone handed us a sample of an ionic liquid, and it changed how we thought about chemical work. 1-Sulfobutyl-3-Methylimidazolium Toluenesulfonate belongs in that revolution.
Researchers turn to this compound for its ability to dissolve both polar and non-polar molecules. That small trick makes it powerful for extracting valuable materials from complex mixtures, breaking up cell walls in green chemistry, or even capturing carbon dioxide. These uses may sound abstract, but they matter: labs searching for greener, safer alternatives don’t need to resort to flammable or toxic organic solvents if compounds like these fill the gap.
Cleaner Energy and Better Batteries
The world keeps talking about electric cars and renewable energy, but batteries still lean on dangerous, polluting solvents. This ionic liquid, thanks to its stability and conductivity, steps up as a better choice for electrolytes in modern batteries and supercapacitors. Chemists see better safety records, longer shelf lives, and improved performance. That matters for everything from grid-scale storage to your cell phone lasting through a busy day.
It’s not just about batteries. Electrochemistry research uses this compound for efficient electrodeposition, meaning it helps form even layers of metal—critical for electronics or specialized coatings. These processes avoid the fumes and waste streams that plague traditional recipes.
Improving Pharmaceuticals
Drug makers run into tough problems with solubility and purification. This ionic liquid can dissolve a wide range of molecules, opening up options for separating difficult compounds or stabilizing sensitive ingredients. I’ve seen researchers extract natural products that simply wouldn’t behave in water or alcohol-based solutions. Using safer liquids eases the burden for both people who work in the factories and people who take finished medicines.
Many green chemistry experts hope that as regulations tighten on toxic solvents, compounds like this will play an even larger role. While ionic liquids aren’t magic bullets, they do cut down on flammability, lower the risk from volatility, and open up possibilities for turning waste into valuable products.
Looking Forward
Scaling up new chemistry always comes with growing pains, but the potential is clear. With better ways to make, reuse, and dispose of these fluids, manufacturers could solve a slew of environmental headaches. More research on the long-term safety and cost will help. If investors and policymakers back the switch, 1-Sulfobutyl-3-Methylimidazolium Toluenesulfonate could move from experimental labs to a starring role in cleaner production, energy, and medicine.
Getting It Right Saves Headaches Later
Years ago, I worked in a warehouse where someone ignored the storage label on a pallet of food additives. The chemical inside caked up, and pretty soon, customers were calling about product malfunctions. Lesson learned: basic details—like how to store and handle sensitive goods—matter a lot. When thinking about a product’s journey, from manufacturer to end user, safe storage and careful handling shape both quality and safety.
Temperature and Humidity: Not Just Fine Print
Too many folks see warnings like “store in a cool, dry place” as just another checkbox. But even a difference of five degrees can shift how chemicals behave. For a pharmaceutical powder, excess moisture may lead to clumping or breakdown of active ingredients. Dryness isn’t about comfort, it’s about blocking out chances for mold or slow chemical reactions that change what you’re selling. A food-safe desiccant can help, but monitoring is key. Without regular checks, a little excess moisture damages entire shipments.
Light Exposure: Why a Dark Room Isn’t Overkill
Sunlight isn’t friendly to every product. Ultraviolet rays can degrade some plastics, fade dyes, and even reduce medication potency. Growing up, I’d find old bottles of antibiotics in the kitchen window, their labels faded and their potency questionable. It’s a simple principle—keep products away from direct light unless packaging guarantees protection. Warehouses using clear roof panels should reexamine their storage strategy or invest in blackout covers.
Safe Stacking and Spill Prevention
Handling requirements go out the window when workers rush or ignore stacking guides. I watched bags of fertilizer spill because they rested four high when two would have sufficed. Guidelines aren’t written just for insurance adjusters. Heavy items on the bottom, lighter up top, and don’t push load limits listed by the manufacturer. Spill kits at the ready, proper labeling, and regular walk-throughs make the difference between a routine day and an expensive clean-up.
Air Circulation and Odor Transfer
A stuffy warehouse isn’t only unpleasant, it affects some chemicals. A musty environment accelerates certain breakdowns, especially with organics and food products. Good vents and fans, spaced shelving, and rotating older stock forward are practical steps. You can smell the difference between a well-run space and one that’s suffered neglect. Over time, odors transfer, which leaves lasting impacts on food and cosmetic supplies.
Training: The Overlooked Factor
Procedures and memos don’t help unless people follow them. New hires at my old job had a habit of skipping “redundant” steps—until management started tailored, hands-on walkthroughs. Product safety culture starts with leadership. Rewarding staff for catching issues, and simple quizzes on proper storage, helped sharpen habits and prevent corner-cutting.
Real-World Fixes Bring Real Results
Problems slice into profits and erode trust when companies see these safeguards as optional. Auditing storage space, updating environmental controls, and hands-on training save money and reputation down the road. Relying on checklists alone leaves too much room for error. Strong processes, clear accountability, and a willingness to adapt are what keep products—and the people who use them—safe.
Stepping Into the World of Ionic Liquids
Find yourself in a laboratory stocked with chemicals, and odds are you’ll spot something like 1-sulfobutyl-3-methylimidazolium toluenesulfonate. This mouthful of a compound falls under the growing family of ionic liquids. These aren’t just fashionable in green chemistry labs. Industries see their potential in extraction, synthesis, desalination, and more. Anyone interested in chemicals dreams big about the claims: less flammable, low volatility, and often less harsh than traditional solvents. But labeling something as "greener" or “innovative” often masks its true risks. That’s where skepticism helps.
Looking at What’s Known
Digging through safety data sheets, there’s not much official hazard labeling attached to 1-sulfobutyl-3-methylimidazolium toluenesulfonate. This lack of warning symbols on bottles in the stockroom doesn’t automatically translate to “safe”—it can also show how fresh, specialized, or niche the compound remains compared to big names like benzene or formaldehyde. Studies investigating acute toxicity or chronic exposure don’t give up much detail yet.
Many ionic liquids show relatively low vapor pressure. With this trait, they don’t easily become airborne, lowering the risk through inhalation. You’re more likely to get it on your skin or mix it into a solution by hand. A few publications suggest that similar compounds in the imidazolium family can irritate skin and eyes. Some experiments hint that at moderate to high doses, there may be reproductive or developmental toxicity in animal tests. Another thing I’ve watched over the years with ionic liquids—what happens in a zebrafish tank or a Petri dish usually shouldn’t be dismissed. Even low toxicity in one area sometimes means persistent, slow buildup elsewhere.
Paying Attention Without Panicking
Often, the newness of an industrial chemical means its full risk profile remains a work-in-progress. In research settings, it’s routine to treat all unknowns with respect: gloves on, goggles tight, lab coat buttoned. Just because there isn’t an alarming report or regulatory red flag yet, that doesn’t give anyone a free pass to splash ionic liquids around without caution.
Experience in labs tells me many chemical injuries happen not from what’s considered highly toxic but from carelessness with substances thought to be “safer.” A simple slip in handling, a spill you meant to clean quickly, and suddenly you’ve introduced an unknown into your own environment—sometimes with headaches or rashes, sometimes with nothing obvious at all.
The Real-World Questions
If you’re working with 1-sulfobutyl-3-methylimidazolium toluenesulfonate, a few practices matter—safe storage away from heat or moisture, clear labeling, and not mixing it into local water supplies. Disposal can’t be an afterthought. As ionic liquids gain traction, we should demand better long-term studies. Industry has the responsibility to fund transparent research—peer-reviewed, out in the open, not just internal memos.
Most importantly, anyone using or surrounded by specialty chemicals has the right to know about substance risks. Whether you’re a student, a professional chemist, or a curious neighbor living near a chemical plant, nothing beats knowledge and vigilance. Technology and innovation charge ahead, but safety can’t be an afterthought in that race.
Bringing Lab Knowledge to Real Life
Ask any chemist, and practical experience always finds its way into the talk about molecules. Chemical structure shapes everything in research and production. In school, I kept seeing the same diagrams in textbooks: hexagons for benzene, lines for bonds. It looked a lot like abstract art, until I handled a vial of pure benzene myself. Things changed. The structure on paper finally matched the smell, the caution, and the rules needed to stay safe around a liquid that evaporates fast and carries more risks than you’d guess.
Molecular Weight—More Than Just a Number
Molecular weight tells a story. It’s not just a sum. Take water (H2O), simple enough: two hydrogens and one oxygen, tallying up to about 18 grams per mole. Measure out that much in a real beaker, it looks like a small splash. Jump to something bigger, like caffeine, with a molecular weight near 194 grams per mole. Suddenly, the same “mole” gives a pile of white powder that smells faintly like your morning coffee. This number helps companies decide shipping costs, pill size, and even customs declarations. Ignore molecular weight, and labs overspend or miscalculate doses. Proper knowledge keeps labs efficient and safe.
The Power of a Structure
Drug discovery taught me not every arrangement of atoms is equal. Isomers might share the same formula—like glucose and fructose, both C6H12O6—but their bodies behave differently. Our enzymes, tiny chemical machines, read structure the way a lock reads a key. Swap an atom’s position or reverse a group, and that key fails to fit. Many times, a patient’s outcome hinges on that difference.
Lower costs push some companies to skip detailed tests, trusting suppliers too much. I’ve seen product recalls thanks to unknown impurities. One small change, invisible without structural insight, brings lawsuits and public outcry. Strict attention to structure keeps trust and safety intact. Regulators set tough standards for a reason—they know shortcuts cause damage nobody wants.
Solving the Unknowns
Confusion grows when documents hide key facts. I’ve watched researchers spend days chasing missing diagrams or partial molecular formulas. They waste time, rerun experiments, repeat tests, and stocks run low. Labs low on funds cut corners, missing tests or settling for low-grade chemicals. Such mistakes risk people’s lives in medicine production. Full data from suppliers—structure, molecular weight, impurities—cuts down confusion and speeds real work.
Making Reliable Data Standard Practice
Real change comes through shared responsibility. Vendors must publish chemical structures and complete molecular weights; labs need to double-check with reliable analytical tools, like NMR and mass spectrometry. Teaching juniors to value these details builds habits for life. Software tools now flag inconsistent data, but trained eyes catch what software can’t. In my work, confirming even “basic” molecules has stopped repeated costly errors. Regulatory agencies and buyers should prioritize suppliers who post and certify accurate, complete molecular descriptions. Reliable data helps everyone avoid surprises that damage both health and business.
In the end, understanding chemical structure and molecular weight shapes safe and effective science. Ignoring them only leads to mistakes nobody can afford—not just in the lab, but for the people who depend on the results.
Getting Real with Laboratory Safety
Anybody who has ever worked in a lab knows that spills happen. There’s no perfect track record in handling chemicals, not even when care levels run high. Some compounds belong to that stack of hard-to-pronounce but common lab chemicals like 1-Sulfobutyl-3-Methylimidazolium Toluenesulfonate. If you’ve used modern ionic liquids, this name probably rings a bell. One time, I watched a graduate student knock over a bottle with a backpack swing — a splash on the floor and across nitrile gloves. Stuff like this snaps folks awake, but comfort with routine can lead to lapses.
The Hazards in Simple Terms
Information from safety data sheets tells us quite a bit. This compound doesn’t behave like regular table salt; it’s not entirely harmless to skin or the airways. Inhalation triggers irritation fast, and so does skin contact, especially if cuts are involved. Eye exposure feels like sandpaper. ScienceDirect and the European Chemicals Agency list potential toxicity issues that deserve respect, not just a shrug. I’ve seen people assuming all ionic liquids act the same, but it pays to check toxicity tables and environmental impact notes. Some stick around in wastewater a long time, and others bring acute hazards.
Spill Response: Small Actions, Big Impact
Spills of this compound present a classic hazard versus routine dance. Fumbling for powder isn’t uncommon, yet the right reaction makes a difference. Grab absorbent pads — plain paper towels aren’t up to the job — and sweep the area with enough ventilation to clear any vapors. If you have a chemical spill kit tucked away, now’s the moment to pull it out and get to work.
Some folks just want to mop up and keep moving, but the floor needs more than a wipe. Labs I’ve worked in always mark spill sites until cleaned with soap and plenty of water, because residues linger. Skipping this step has led to slip-and-fall stories and repeat contamination. Gloves, eye protection, and a decent lab coat mean the difference between mild discomfort and a full clinic visit. If anything lands on skin or clothing, rinse right away — no exceptions. Fume hoods aren’t just furniture; if the stuff goes airborne, experts hit the hood or step outside.
Exposure: Don’t Wait and Wonder
Nobody enjoys reporting an accident, but silent exposure saves no one. Wash the affected area with gentle water flow for minutes, and always remove contaminated gear. Years ago, I ignored a tiny splash on my forearm, and the rash lasted a week. Going to occupational health after a mishap might feel dramatic, but chemical safety isn’t about courage or saving time — it’s about avoiding long-term consequences. Even small exposures sometimes build up, especially in tight spaces with shaky ventilation.
Better Systems, Better Outcomes
Training means more than rattling off protocols. Facilities with regular drills and quick-access clean-up kits see fewer close calls. Some universities and chemical plants have started tracking near-misses and rewarding quick reporting to break the “don’t blame me” silence. Routine reviews of ventilation and storage also reveal hidden risks. Investing in real, hands-on practice means more than decorations on the wall. Nobody wants to be the story others use in future safety lectures.
New developments in ionic liquids make for exciting research, but respect for safety gives everyone a chance to go home with nothing worse than sore feet. Spills and exposures teach lessons quickly — the smart response saves time and keeps trouble small.