1-Vinyl-3-Dodecylimidazolium Bromide: An In-Depth Commentary
Historical Development
Chemists began exploring imidazolium salts early in the last century to tap into their ionic nature and stable physical structure. At first, applications targeted specialized laboratory functions, with research moving steadily as industrial production started to mature in the 1990s. 1-Vinyl-3-dodecylimidazolium bromide entered the scene as interest in room-temperature ionic liquids and functionalized polymers picked up. Scientific literature soon started referencing unique dodecyl side chains, noting their remarkable impacts on solubility and micelle formation. As more groups experimented with derivatization, the focus shifted from imidazolium core chemistry toward sidelong opportunities in advanced materials, membranes, and catalysis. Pioneers in this area drew on earlier breakthroughs in alkylation and ionic liquid handling to broaden the scope and push for broader accessibility.
Product Overview
Among the family of ionic liquids and functional cationic surfactants, 1-vinyl-3-dodecylimidazolium bromide stands out with its eclectic vinyl group and emphatic twelve-carbon chain. This composition supports flexibility for further derivatization, including potential for polymerization and self-assembly. The product comes as a solid, usually off-white or pale yellow, and unlike many short-chain surfactants, handles complex organic-inorganic interfaces with greater tenacity. Suitably stored in air-tight containers, this compound resists ordinary degradation, which means research groups working in both academic and development environments can count on its integrity throughout extended study periods.
Physical & Chemical Properties
This compound blends high ionic conductivity with low volatility, properties derived directly from its imidazolium core and bromide counterion. Its melting point commonly lies above room temperature, yet below the boiling thresholds found in traditional ionic salts. The dodecyl side chain imparts greater hydrophobicity, which causes notable phase separation in aqueous environments yet encourages micellar and vesicle formation in specialized solvents. The vinyl group broadens the reactive palette, supporting grafting onto polymeric backbones through simple free-radical approaches. The material’s viscosity in solution and its tendency to stabilize emulsions position it as a productive agent in multiple surfactant applications. Researchers tracking NMR and FTIR spectra can identify clear signals indicating robust imidazolium bonding and modest reactivity at extremes of heat and pH.
Technical Specifications & Labeling
Technical data sheets for 1-vinyl-3-dodecylimidazolium bromide usually report purity levels above 98%, with water content remaining minimal through careful synthesis and drying. Specific density ranges hover between 1.05 and 1.10 g/cm³, with solubility reported as high in polar organic solvents and selective in deionized water. Labeling standards set by GHS and REACH govern packaging and storage requirements, including hazard pictograms for potential skin and eye irritation, as well as preventative guidance for spills. Batch information from reliable producers always includes full chemical structure, molecular weight (around 381 g/mol), and recommended handling procedures. For research-grade supply chains, material safety data sheets supplement technical sheets with guidance for controlled substance management and accident response.
Preparation Method
Industrial synthesis of this compound typically begins with 1-vinylimidazole and dodecyl bromide. The reaction proceeds under anhydrous conditions to drive alkylation at the nitrogen-3 position, requiring careful temperature management to suppress side-reactions or polymerization. Excess dodecyl bromide often helps ensure complete conversion and simplifies downstream purification. After the reaction finishes, the product crystallizes upon cooling, then undergoes filtration and repeated washing, usually with non-polar solvents to strip away unreacted alkyl halide and organic byproducts. Final drying under reduced pressure brings the compound to stable, anhydrous form. Analytical checks, like chromatography and spectroscopic fingerprinting, confirm product identity and rule out contamination.
Chemical Reactions & Modifications
Beyond its role as a building block, 1-vinyl-3-dodecylimidazolium bromide lends itself to free-radical and cationic polymerization due to its vinyl group. Laboratory teams can react it with styrenic or acrylate monomers to create tailored polyelectrolyte brushes, responsive membranes, or dynamic coatings. The lengthy dodecyl tail encourages self-assembly in selective environments, and creative teams have shown its effect on stabilizing silica nanoparticles and forming hybrid organic/inorganic composites. The imidazolium head allows further modification, including ion exchange to swap bromide with alternative anions such as bis(trifluoromethylsulfonyl)imide or hexafluorophosphate, shifting hydrophobicity and thermal stability. More recently, researchers have investigated functionalizing the vinyl group with thiol-ene click chemistry, expanding uses in soft-matter and flexible electronics research.
Synonyms & Product Names
Chemical suppliers recognize this compound by several names, including 1-vinyl-3-dodecylimidazolium bromide, 3-dodecyl-1-vinylimidazolium bromide, and VIIM-Br. Product listings occasionally reference its role as a surface-active ionic liquid or polymer precursor, reflecting its dual laboratory and industrial status. Some catalogues distinguish the compound by its CAS number (used for cross-referencing across regulatory databases). Naming conventions may also shift slightly in journals, leading to alternating ordering of substituents—a point of confusion, yet an easy troubleshooting target for product managers and procurement staff.
Safety & Operational Standards
Wearing gloves and goggles isn’t just a box to tick off when getting started. Skin exposure may cause some irritation, and inhaling dust or working in poorly ventilated places could bring trouble over time. Good practice means working with localized fume extraction, sealed weighing, and prompt spill cleaning, because moisture not only jeopardizes compound quality but also accelerates hydrolysis that messes with analytical controls later in a project. Safe disposal goes beyond tipping leftovers in a sink. Transfer solutions to clearly labeled containers for collection under hazardous waste protocols, following both university and state mandates. Those who end up in emergency rooms after contact without PPE remind us that nothing replaces old-fashioned caution, even with cleaner labels and modernized MSDS sheets.
Application Area
People use this compound well beyond the boundaries of niche synthesis. It finds a place in the creation of robust, conductive polymers, powering advances in battery electrolytes and sensor development. Its surfactant capabilities shine in emulsion stabilization for paints and coatings with exacting specifications. Companies working on antimicrobial surfaces experiment with the long alkyl tail, seeing how such molecules disrupt microbial membranes and improve environmental hygiene. In separation technologies, the ionic and hydrophobic duality supports membrane selectivity, helping address challenging resource purification projects from water desalination to heavy metal scavenging. Industrial labs turn to its customizable structure for antistatic and self-healing materials, while university teams test it for enhanced catalysis and as a carrier for drug delivery—showing the breadth of real-world impact.
Research & Development
New papers appear every year pushing this compound into unfamiliar territory. Material scientists examine how its properties alter polymer electrolyte landscapes, boosting ion mobility without sacrificing safety. Chemists focused on green process engineering consider how imidazolium-based surfactants replace more hazardous options in extraction schemes. Engineers designing next-generation electronics value the partnership between high molecular weight and manageable processability, opening up flexible device templates. Interdisciplinary groups draw competitive funding by showing how tailored modifications on this structure give rise to targeted antimicrobial membranes or improve fluorescence in imaging and diagnostics. The drive to mitigate toxicity and environmental footprint inspires labs to create bio-based analogs or rework the structure for eventual breakdown in nature, balancing innovation with responsibility.
Toxicity Research
Many newer chemicals raise more questions than answers on human and environmental safety, and 1-vinyl-3-dodecylimidazolium bromide is no exception. Early cell culture tests showed low-level toxicity to a range of microorganisms and murine cells, mainly linked to disruptions in membrane integrity from the dodecyl tail. Acute aquatic toxicity studies reveal significant challenges for wastewater treatment, particularly as even small leaks compromise microbial functioning. With rodents, longer-term exposure leads to liver and kidney stress at high doses, echoed in studies on structurally similar imidazolium compounds. This isn’t simply a laboratory worry—manufacturing and downstream use produce effluent streams that demand real monitoring to avoid chronic buildup in sensitive ecosystems. Many teams advocate for careful hazard labeling and enhanced personal and environmental safety measures, recommending substitution or chemical trapping where lower-toxicity alternatives function as well.
Future Prospects
The road ahead for this compound looks both wide open and riddled with speed bumps. Green chemistry goals keep urging researchers toward less persistent, more easily recycled molecules, yet its modularity and reactivity invite non-stop tinkering by polymer chemists and engineers seeking just the right property set. Markets tied to renewable energy, high-performance electronics, and sustainable materials keep driving demand for adaptable surfactants, ionic conductors, and functional, antimicrobial surfaces. At the same time, regulators push for better tracking of novel ionic liquids throughout their lifecycle, so the smart money invests in degradable analogs or technologies that streamline recovery and reuse from mixed waste streams. People who bridge synthesis, toxicology, and product design will shape not only new applications but also the protocols that ensure safety and responsibility accompany every breakthrough.
A Unique Ingredient in Modern Chemistry
Some chemicals stand out because of the roles they play across several fields. 1-Vinyl-3-dodecylimidazolium bromide falls into that category. This compound grabs the attention of researchers as an ionic liquid, a material that doesn’t act like most salts. It stays liquid at room temperature, even with its quirky structure, which includes a lengthy dodecyl chain and a vinyl group attached to an imidazolium ring. That combo brings out surfactant power, distinct conductivity, and chemical stability.
The Real-World Utility: More Than a Lab Experiment
Plenty of chemists reach for this ionic liquid when they look for specialty solvents. Its molecular structure helps dissolve both organic and inorganic substances, which comes in handy during those moments when water or typical oils just don’t cut it. Think about the synthesis of advanced polymers—researchers count on 1-vinyl-3-dodecylimidazolium bromide for the direct polymerization method. This lets them build complex chains that find their way into advanced coatings, adhesives, and even the surface of medical devices.
In environmental science, this compound makes a difference during separation processes. Its unique ability to draw certain contaminants out of water sets it apart. Wastewater treatment labs have experimented with removing dyes, heavy metals, or pharmaceutical residues using ionic liquids like this one. There’s something satisfying about seeing practical chemistry in action—watching clean water flow out at the end feels like more than just science; it feels like a win for the community.
Challenges in Daily Use
Lab routines can get messy with exotic materials. Some colleagues worry about handling ionic liquids, because their health effects don’t always come with long histories. Researchers pull on gloves and work under a hood, but the lack of years’ worth of toxicity data keeps everyone alert. Questions about environmental persistence spark debates around the office coffee machine. Everyone cares about scientific progress, but nobody wants to see the planet pay the price.
Cost pops up as another roadblock. 1-Vinyl-3-dodecylimidazolium bromide often isn’t a bulk commodity. Sourcing it for scaled-up industrial work takes some hunting and planning—a reality for anyone managing a research budget.
Looking Toward Smarter Chemistry
I’d like to see more funding go toward toxicity studies and environmental impact reports for these new chemicals. Open publication and sharing data helps build a collective safety net. If we know what to expect, labs can design better handling protocols, while manufacturers work on greener synthesis routes. The movement toward “green chemistry” has already started influencing how chemists select ionic liquids. Efforts now focus on designing versions that perform well in the lab and break down harmlessly after use, closing the loop.
Staying informed about advances like 1-vinyl-3-dodecylimidazolium bromide matters to more than just researchers and students. The breakthroughs bubbling out of these labs often feed into the products and clean technologies we see years later. Paying attention now helps make sure the benefits don’t come with hidden costs in the future.
Getting Familiar with the Building Blocks
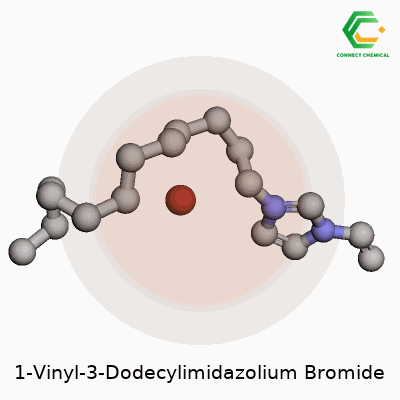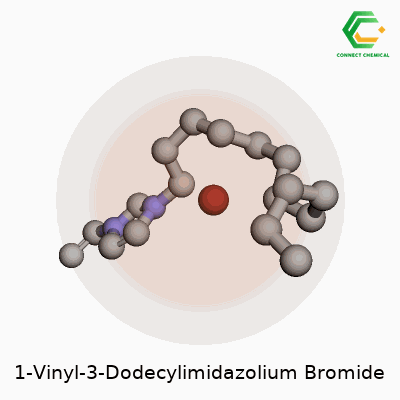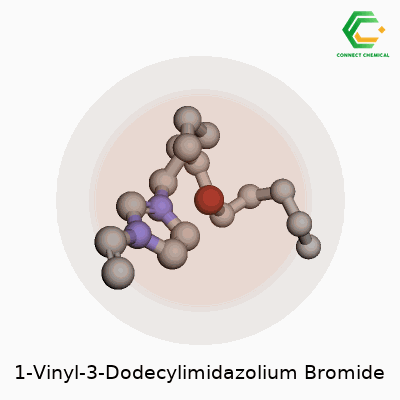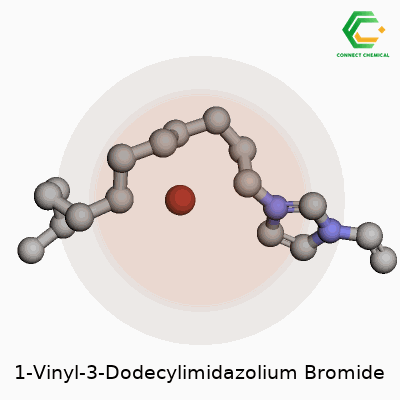
Ever looked at a chemical name and thought it sounded more like a riddle than something you might find in a lab? 1-Vinyl-3-dodecylimidazolium bromide falls into that camp. You start picking it apart and you start to see some parts you might know. There’s the “imidazole” at the core, a five-membered ring with two nitrogen atoms. Toss in the “vinyl” group at one end—a two-carbon side chain with a double bond—and a “dodecyl” group, which counts out to a twelve-carbon straight chain stuck onto the other nitrogen. Finish things off by balancing the positive charge on the ring with a bromide anion.
Why This Compound Draws Attention
Most people don’t linger on the way a long alkyl chain can change the way a molecule acts, but add twelve carbons to an imidazolium ring and you shift things in a big way. This isn’t random: chemists pick long alkyl chains for their tendency to make molecules less soluble in water and better at dissolving oily stuff. Now sprinkle on that vinyl group, and you open up the possibility to use this as a monomer—something you can snap together into something bigger through polymerization.
With these tweaks, this molecule finds itself a candidate for ionic liquids, those curious salts that remain liquid at room temperature and have a well-earned reputation for helping green chemistry move forward. Many chemists use imidazolium-based ionic liquids to replace noxious organic solvents, avoiding the headaches that come with high volatility and toxicity. 1-Vinyl-3-dodecylimidazolium bromide’s structure does a lot of heavy lifting for researchers hunting better ways to extract, separate, or catalyze reactions.
Connection With Real-World Applications
Structure unlocks job potential. That twelve-carbon tail helps form micelles and can even help trap hydrophobic contaminants, leading some folks to test this salt for removing pollutants from water. Material scientists use that vinyl group, making polymers that find their way into everything from smart coatings to specialty membranes. In battery research, the low volatility and good ionic conductivity of these salts promise safer and sometimes more efficient energy storage.
As more industries set goals to lower emissions and stretch the limits of efficiency, compounds like this one step forward. Chemists already eye the structure for use in drug delivery systems, tapping that long hydrophobic chain and the ionic nature to shuttle molecules across membranes.
Taking Responsibility and Safety Seriously
Learning about chemical structure brings up plenty to celebrate, but it comes with serious duty. Halide counter ions like bromide can beware of leaching into the environment, and long alkyl chains sometimes don’t break down fast. Putting green claims through real-world tests takes time, so researchers keep a close watch on toxicity and degradation.
Not every lab has the tools or safety training for handling ionic liquids. More sharing between chemists and environmental specialists helps avoid the trap of solving one problem while causing another. The push for biodegradable ionic liquids keeps gaining ground, and more transparent reporting of life cycle impacts helps keep innovation honest.
Looking Ahead
By loosening the secrecy around molecular tweaks and sharing failures along with wins, chemistry can keep improving. Real-world needs demand more than clever synthesis—they call for humble review and practical transparency. Sharing these stories doesn’t just build better compounds; it builds trust between labs, companies, and communities relying on safer science.
Why Careful Storage Matters
Anyone who has ever worked with specialty chemicals, like 1-Vinyl-3-Dodecylimidazolium Bromide, knows how crucial it is to keep things safe and straightforward. This compound serves as an ionic liquid in advanced labs and industrial settings, and safety begins with how you store it. Keeping it dry and shielded from moisture can’t be overstated. Even a small amount of humidity creeping into a container could spoil the product or, worse, create hazardous conditions. You only have to look at corrosion or degraded reagents to see how water and the wrong air mix spell disaster for a chemical storehouse.
Daily Storage Precautions
For storage, a tightly sealed container in a cool, well-ventilated spot proves its worth. Plastic bags or open vessels set on a dusty shelf just invite headaches. People often forget: some specialty chemicals, including quaternary imidazolium salts, break down when exposed to too much heat or light. Even a few hours of sunlight near a window start changing what’s in the bottle. This isn’t just a theoretical problem. I remember a lab tech telling me once about a sample left too close to a radiator; the results never matched expectations again, and the shelf-life of the product dropped to zero.
Check the seals, keep away from strong oxidizers, acids, and bases. If you try storing it near reactive chemicals, you increase the risk of chemical reactions that nobody wants to happen by accident. Cross-contamination remains a risk in crowded labs. Even dedicated labeling and storage cabinets can’t replace diligence. Sticking to single-use scoops or spatulas helps, and avoiding the temptation to dip into stock containers “just for a sample” preserves the rest for future use.
Safety Isn't a One-Time Effort
1-Vinyl-3-Dodecylimidazolium Bromide can be an irritant, both to skin and eyes. Standard lab PPE—nitrile gloves, goggles, and a lab coat—serve better here than wishful thinking. Small spills, once ignored, turn into big problems by contaminating workspaces. I’ve seen colleagues dismiss powder dust as harmless, but later regret it when it gets blown into an instrument or pulled into an unnoticed corner of the bench.
Using a fume hood pays dividends. Even if this chemical doesn't off-gas like solvent, handling many powders this way reduces exposure risk and keeps everything contained. Over time, these habits save resources and, most importantly, keep people healthy. Disposal processes deserve attention too; pouring leftover substances down the sink proves wasteful and environmentally damaging. Many university and corporate labs now have clear protocols on hazardous waste, and not following them can bring audits, fines, or worse.
Improving Lab Practices
Building a routine around regular checks and inventories prevents old or degraded stocks from webbing their way through the storage system. I learned to never trust a handwritten label from years ago—fresh records, digital tracking, and dating each container keep everyone honest and reduce confusion. Training matters as much as any fancy equipment, so bringing new technicians up to speed and running refresher sessions matters for safety as well as efficiency.
Ultimately, the effort invested in proper storage and careful handling reflects a culture of respect, both for the science and for everyone working nearby. These habits don’t demand much, but they repay trust with a safer lab and higher-quality results.
Looking Closely at Laboratory Chemicals
The chemical industry always pushes for new methods and materials, and 1-vinyl-3-dodecylimidazolium bromide belongs to a class of ionic liquids that attract plenty of attention from researchers. This compound offers unique electrochemical properties, and some labs test it for roles in batteries, catalysis, and coatings. It’s never enough to focus on function alone, though. The question about safety, toxicity, and hazards sits right alongside those potential benefits.
What’s On the Label?
Let’s get straight to real-world data. Researchers haven’t gathered mountains of evidence about its effect on people or the environment. What we do know: many ionic liquids, especially those with long alkyl chains, behave differently from traditional organic solvents. Some break down slowly and resist water-based dilution, raising flags for aquatic toxicity and bioaccumulation. The dodecyl group, for instance, is known to impart higher toxicity in living organisms compared to shorter chains. This means chemists who handle such substances usually wear gloves, goggles, and coats—both in universities and in industry labs.
What Do Safety Data Sheets Say?
Most safety sheets list 1-vinyl-3-dodecylimidazolium bromide as an irritant. Exposure to skin, eyes, and the respiratory tract might cause rashes, redness, or coughing. Inhalation should be avoided, and accidental contact with eyes leads right to an eyewash station. No safety sheet calls it mutagenic or carcinogenic, but no sheet guarantees that today’s knowledge catches everything that could surface tomorrow.
Environmental Impact Takes Time to Unfold
As an ionic liquid, 1-vinyl-3-dodecylimidazolium bromide does not evaporate quickly compared to volatile compounds. This might seem harmless at first because less vapor means less inhalation risk. Yet slow degradation in soil and water invites long-term problems. A study from 2020 tested related chemicals and found some caused harm to freshwater shrimp and fish at fairly low concentrations. No one wants new tech to bring along another DDT story, so extra caution makes sense.
Real Experience with Precaution
My time in academic labs taught me that any compound, novel or old, demands respect. We never shrugged off unknowns because often the field uncovers effects only after years of use. Students, professors, and industry staff know how easy it can be to overlook a chemical in the rush to try a new experiment. Once, a mishandled spill of a seemingly mild organic salt sent a colleague to urgent care with a chemical burn. This memory brings new relevance every time a new ionic liquid lands in my workspace. Taking time to check the latest journals, discussing protocols, and updating waste disposal methods matters far more than racing through experiments. Labs thrive on vigilance and on learning from small incidents before they spiral.
What Can We Do Better?
Scientists and industries would benefit from deeper toxicity testing. Regulatory reviews need to catch up with chemicals like this, so new materials don’t outpace our understanding. Minimal standards on production, storage, and disposal, plus ongoing research on environmental effects, set a higher bar for safety. If information is thin, erring on the side of overprotection costs far less than a remediation project years down the line. As curiosity and innovation drive chemistry forward, transparency about hazards ensures we keep our health, and our planet, at the forefront too.
The Numbers: Molecular Weight and Purity
Anyone spending time at a chemistry bench knows, you can’t just pick a chemical off the shelf without thinking about the numbers printed on the label. For 1-vinyl-3-dodecylimidazolium bromide—a mouthful, but a key ionic liquid—two numbers jump out. The molecular weight clocks in at 389.41 g/mol. Purity, on the other hand, often lands around 98%, though that can shift based on who’s making it. These two facts set the starting line for everything that follows in both academic research and commercial use.
Why Accuracy on Molecular Weight Matters
People sometimes brush past molecular weight, assuming it’s just a detail, but that’s a shortcut that doesn’t end well. Picture preparing a solution meant for a new type of conductive polymer. Mix even a gram off, and the resulting molarity goes sideways. Getting the wrong molecular weight doesn’t just waste time and resources—it can mess with reaction outcomes, create noisy data, or even damage equipment. Over the years, I’ve watched projects grind to a halt simply because someone misread a label. That’s a headache any chemist can relate to. Checking molecular weight directly from the formula—C17H31BrN2—helps keep things on track: add the atomic weights, double-check, and there’s your answer.
The True Cost of Purity
No one expects 100% pure chemicals every time, but purity brings its own set of trade-offs. At 98%, this compound isn’t carrying a ton of baggage, but trace contamination always lingers. If a lab experiment is sensitive to halide impurities or trace imidazole, even that tiny 2% matters. Toxicity studies, sensor tests, or battery research start to wobble if the numbers drift. In my own work, even when a supplier swore by high purity, running a batch through HPLC sometimes showed a surprise: ghost peaks, evidence of impurities, or water picked up from sloppy storage. These slip-ups lead to days chasing gremlins, trying to figure out whether an odd result is a discovery, or just a fluke from subpar purity.
How to Improve Confidence in Your Materials
Researchers and industry folks aren’t powerless here. Vendors offering full Certificates of Analysis—not just stickers—make a difference. I always ask for actual chromatograms if something crucial hangs in the balance. There’s value in running independent purity checks. In my lab, NMR and mass spectrometry are old habits on shipment day, because it’s about trust but verify. Even the best-known suppliers can have off days. Some teams use in-house purification, sparging ionic liquids or recrystallizing, but nothing replaces solid initial specifications. Keep good records, and share experiences—some suppliers have a better reputation for real numbers than others.
Driving Toward Better Standards
This isn’t just an academic issue. As ionic liquids move from the lab into big factories, corners cut on weight or purity become safety issues, not just scientific ones. The movement toward tighter international standards—pushed by both researchers and regulators—pushes all producers to step up. Transparency around those two core numbers, and a willingness to audit, gives everyone downstream a better shot at success.
With all the focus on rational design and high-throughput screening, the basics still count: checking numbers, double-checking purity, and refusing to trust until facts line up. That attitude has saved more than one project—and kept a lot of future problems out of the bottle.