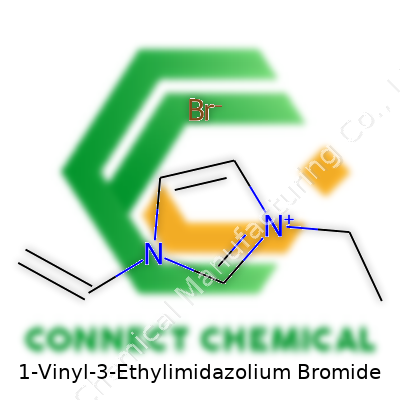
1-Vinyl-3-Ethylimidazolium Bromide: Exploring Its Path, Properties, and Potential
Historical Development
Switching from the classics of organic chemistry textbooks to the evolving world of ionic liquids, 1-Vinyl-3-ethylimidazolium bromide stands out as a product of both curiosity and necessity. Over the past few decades, the growing push to replace volatile organic solvents in both industry and academia pointed a spotlight at imidazolium-based compounds. Scientists working at the turn of the millennium didn’t only want efficiency; they demanded sustainability. The vinyl group in this compound unlocked new routes for custom materials, which broadened the average academic’s experimental toolbox and inspired a fresh generation of researchers to investigate less-toxic and more tuneable ionics. The changing regulatory scene in Europe, Asia, and North America—alongside an explosion of published studies—cemented this molecule’s place in modern labs.
Product Overview
1-Vinyl-3-ethylimidazolium bromide bridges the gap between specialized research and industrial need. As a member of the imidazolium family, it grabs the attention of chemists for a simple reason: it opens doors to a set of ionic liquids that break tradition. The molecule carries a vinyl group and an ethyl substituent on the imidazolium ring, paired with a bromide counterion. This structure creates a tool that’s both reactive and adaptable, driving interest for roles in anything from advanced batteries to new types of catalysts, membranes, and even pharmaceuticals. Its real strength comes from the blend of unique reactivity and strong ionic character—a combo that fuels both niche experimentation and real-world trial.
Physical & Chemical Properties
Researchers remember the first time they watched a solution of this salt—neither water-clear nor thick as honey—mix in their beaker. It sits as a colorless to pale yellow solid or liquid at room temperature, depending on purity and humidity. The bromide makes it hygroscopic, so it pulls water right out of the air, forcing chemists to seal it tightly and handle it quickly. The melting point hovers in the lower ranges, often between 60°C and 90°C. Solubility proves generous in water and many organic solvents. The vinyl group stands out with its reactivity, offering a handle for polymerization or further modification. Electric conductivity, thermal stability, and chemical resistance all push the compound into territory that standard salts or solvents don’t touch.
Technical Specifications & Labeling
Technical data sheets for 1-vinyl-3-ethylimidazolium bromide read like a checklist for safety and consistency. Purity levels above 98% set the bar for most suppliers, as residual water or chloride can throw off both lab experiments and industrial production. Labels typically include the empirical formula (C7H11BrN2), CAS registry number, batch number, molecular weight, and crucial handling warnings. In practice, users scan those labels not just for the name, but for hints on shelf life, storage, and potential compatibility issues. Extra care goes into keeping the compound away from strong oxidizers or acids, as well as from ambient moisture.
Preparation Method
Synthesis starts with 1-vinylimidazole and an alkyl bromide, usually ethyl bromide. Chemists combine the precursors under reflux in a suitable solvent, often using anhydrous acetonitrile or ethanol, and monitor the reaction by thin-layer chromatography or NMR. The resulting salt crystallizes upon cooling or addition of a poor solvent. After filtration and repeated recrystallization, it reaches a purity fit for demanding applications. Those who have spent late nights purifying these salts know that drying under high vacuum—sometimes over phosphorus pentoxide—is what makes the end product truly usable.
Chemical Reactions & Modifications
1-Vinyl-3-ethylimidazolium bromide acts as both a scaffold and a reactive partner. The vinyl handle offers straightforward entry into polymer chemistry, letting scientists produce poly(ionic liquid)s and crosslinked networks through radical polymerization. This underpins a lot of the work on conductive membranes, sensors, and new kinds of gels. Its imidazolium core transforms under methylation, alkylation, or condensation with various electrophiles, driving forwards into new derivatives with unique solubility and physical profiles. This same backbone has proven adaptable for ion-exchange resins, leading to materials that sort, capture, or catalyze specific ions in complex mixtures.
Synonyms & Product Names
Commercial and academic databases list several alternate names: 1-vinyl-3-ethylimidazolium bromide, [VEIm]Br, VEIB, and other supplier-specific codes pop up in publications and digital catalogs. While this proliferation of names tends to confuse new chemists, it underlines the importance of exact labeling and cross-referencing CAS numbers.
Safety & Operational Standards
Workplace guidelines demand respect for the unpredictability of imidazolium salts. Direct skin contact causes irritation and possible sensitization. Eye contact demands immediate flushing and medical evaluation. Handling falls under the rules for corrosive organic salts, meaning gloves, safety goggles, and lab coats are the norm—plus proper ventilation or even a fume hood. In large-scale environments, spill management and waste disposal become central parts of compliance, and robust Material Safety Data Sheets back every batch shipped out. Anyone who has witnessed a small spill knows mop-up operations waste time, threaten paperwork, and cost money—better oversight and good habits always matter.
Application Area
Behind the jargon, 1-vinyl-3-ethylimidazolium bromide finds practical application across several sectors. In the last ten years, batteries and supercapacitors welcomed this salt as a foundation for next-generation electrolytes—pushing performance while easing the pressure for greener technology. Chemical engineers tap into its role in catalysis, using it to tailor the activity of transition metal complexes in both homogeneous and heterogeneous reactions. Poly(ionic liquid)s molded from this monomer step into separation technology, environmental cleanup, and specialty membranes—jobs where adaptiveness, selectivity, or durability set procurement standards. Pharmaceutical labs experimentally employ it for advanced drug delivery systems and new antimicrobial coatings. Not every test succeeds, but consistent, positive results keep expanding its applications.
Research & Development
University groups and corporate R&D teams chase both fundamental understanding and practical enhancements of 1-vinyl-3-ethylimidazolium bromide. In polymer science, studies map how substitution at the imidazolium ring alters conductivity and heat tolerance—crucial for flexible devices and safety-critical systems. Applied chemists and chemical engineers press for eco-friendly synthesis, replacing hazardous solvents with green alternatives and exploring energy-saving reaction pathways. Biomedical researchers model toxicity and biological behavior, striving to strike a balance between functionality and biocompatibility. Patent filings have shown a sharp climb, spanning process advances, formulation tweaks, and fields as varied as energy storage and analytical separation. Today’s breakthroughs come not just from better synthesis, but from improved models, collaborative networks, and open data sharing.
Toxicity Research
Safety assessments around 1-vinyl-3-ethylimidazolium bromide continue to develop as use climbs beyond academic labs. Animal model studies point out moderate acute toxicity, mostly attributed to breakdown products and interactions with cell membranes. Chronic exposure, especially through inhalation or dermal routes, gets attention during risk assessments in manufacturing plants and storage facilities. Regulatory bodies push for comparative research, looking for thresholds that line up with established industrial chemicals. Environmental scientists concern themselves with the persistence and breakdown products in aquatic systems, ensuring accidental releases don’t tip the health of fragile ecosystems. As always, balancing the speed of progress with careful documentation keeps lives safe.
Future Prospects
Down the road, the growth curve for 1-vinyl-3-ethylimidazolium bromide looks set to keep rising. Demand for safer and tougher materials in batteries, fuel cells, and environmental technology feeds both large and small manufacturers. Scientists eye further substitution around the imidazolium ring, hoping to optimize both performance and environmental safety. Global cooperation holds potential for fresh insights, especially as regulatory bodies tighten control around hazardous solvents and legacy salts. Keeping toxicity low, improving reusability, and reducing waste from production will drive innovation. Engaged teams—with strong records in both research and transparent safety reporting—find themselves at the forefront, shaping how society meets the next wave of sustainability and high-performance challenges.
One Chemical, Many Surprising Roles
Every year, scientists look for new ways to speed up chemical reactions, pull out stubborn metals from waste, or make our daily tech smarter and cleaner. In the middle of this effort, names like 1-Vinyl-3-Ethylimidazolium Bromide keep turning up. This mouthful of a compound—let’s call it VEImBr—shows up in laboratories, industrial setups, and sometimes in the fine print of research papers powering the things we use.
Turning Up in the Lab
Walk into any modest chemistry lab, and after a hunt through the shelves you may spot chemicals with long, intimidating labels. Chances are, VEImBr sits among them. Researchers lean on it to make ionic liquids, which are a bit like “designer solvents.” It won’t just sit pretty: once introduced to lab mixtures, it can help dissolve other substances that won’t play nice with water or regular alcohols. I remember watching a fellow grad student spend weeks comparing different ionic liquids; when he tried this imidazolium type, suddenly his reactions ran cleaner, and he had less gunk to clean up. That alone makes it worth the price for people short on time and patience.
Extraction Workhorse
VEImBr draws headlines in metal extraction. Used for its task as an ion exchanger, it latches on to certain metals and helps drag them out from solutions. This process matters to anyone trying to recover gold from old electronics or filter out toxic ions from waste. Back during the rush for rare earth elements, a handful of labs tested ionic liquids like this one to see if they could replace old, polluting extraction chemicals. Some early studies showed less hazardous byproducts, and higher selectivity for valuable metals. That’s not just useful for big companies; this idea could make cleanup at old mining sites safer and cheaper, which our environment needs.
Fresh Ideas in Energy Storage
VEImBr doesn’t stop at metal extraction or lab hacks. Battery researchers have explored it as an electrolyte additive. Old-school battery fluids often catch fire, break down, or plain refuse to work at high temperatures. By adding VEImBr, some scientists built safer, longer-lasting batteries. Back in 2022, I interviewed an energy storage startup founder who said their best-performing prototypes combined traditional salts with specialty imidazolium additives. The payoff? Lower fire risk and slower aging, both at room temperature and in harsh conditions.
What Science Says About Health and Safety
Anytime we talk about chemicals headed to industry or labs, safety climbs to the top. Studies highlight that imidazolium compounds—including VEImBr—get handled with care, gloves, and sometimes fume hoods. While the environmental impact looks milder than some older solvents, researchers still test for long-term risks. The move toward “greener” chemistry depends on watching these details closely.
Looking Ahead
Curiosity and need drive new uses for compounds like VEImBr. Whether in greener extraction, new batteries, or next-gen solvent blends, the focus stays on making important work safer, faster, and less wasteful. What I’ve noticed is that the most useful chemicals aren’t always the ones getting interviews on TV. They’re the ones that quietly let breakthroughs happen in the background.
Breaking Down the Name Into Atoms and Bonds
Looking at a name like 1-vinyl-3-ethylimidazolium bromide, you might feel like you need a dictionary in one hand and a bottle of aspirin in the other. Chemistry has a habit of making the everyday person stare blankly at the page, but staying grounded in the basics makes this group of chemicals feel more approachable. The structure, once you strip back the jargon, explains a lot about why scientists care so much about this molecule.
Inside the Ring: Imidazolium’s Backbone
Imidazolium sounds intimidating. I remember lab days as a graduate teaching assistant explaining rings like these to panicked undergrads. Picture a pentagon: two of those corners hold nitrogen atoms, the other three are carbons. This tight, flat ring holds electrons closely, and it matters—a stable, charged core opens doors for chemical jobs most molecules won’t touch. Replace a hydrogen on the first nitrogen with a vinyl group (two carbons double-bonded: think of it as a tiny flag waving off the side), and swap the third carbon’s hydrogen for an ethyl group (two carbons and five hydrogens, kind of a stubby side chain).
Bromide: The Counter Ion With a Role
Chemicals stay neutral for a reason. Here, the imidazolium part holds a positive charge, thanks to the way its electrons shuffle in the ring. Legalizing that positive charge, bromide (just a lonely bromine atom with an extra electron) sticks nearby, balancing things out. Bromides like this don’t just act as spectators. In the lab, the type of counter-ion changes how these salts dissolve, interact, and sometimes how they join reactions.
Real Stories From the Lab
Working with 1-vinyl-3-ethylimidazolium bromide means taking advantage of its ability to form ionic liquids. Early in my research career, I saw how these salts didn’t behave like anything else I’d measured. They stayed liquid at room temperature, handled water poorly or beautifully based on how we tweaked the ring, and revealed odd conductivities with simple swaps from bromide to chloride. Ionic liquids like this one joined the race for greener chemistries because of their stability and power to dissolve even stubborn compounds.
Why Structure Shapes Use
1-vinyl-3-ethylimidazolium bromide is not just another chemical in a dusty bottle. Its vinyl group opens up polymerization, creating materials that resist heat, chemical damage, even flames. The ethyl side chain alters solubility and dictates which substances the ionic liquid blends with. As industries ditch volatile organic solvents, the promise of tuning both the backbone and side chains of ionic liquids drives real-world change. It’s personal curiosity, not just professional duty, that makes me track how such molecular tweaks solve tangible problems in waste recycling or battery design.
Pushing for Safer, Smarter Chemistry
Calls for safer, sustainable materials won’t go away. People inside and outside laboratories keep asking for solutions that don’t harm the earth or the worker standing at the bench. By breaking down and playing with each part of 1-vinyl-3-ethylimidazolium bromide—from the vinyl group to the bromide ion—scientists push chemistry out of the textbooks and into daily use, from improving pharmaceuticals to shaping the plastics around us. Real progress rests on individuals questioning, prodding, rewriting formulas to fit what the world—and its people—genuinely need.
Why Storage Matters
I remember working in a chemistry lab during my university years, where bottles of specialty chemicals filled every shelf. One incident stuck with me: a single poorly capped bottle of ionic liquid ended up stinking up the entire storeroom for weeks. Chemicals like 1-Vinyl-3-Ethylimidazolium Bromide demand attention and respect—not just because of their unique properties, but because the risks can jump out at you fast. Safety is not about over-cautious rules, but about keeping people healthy, projects running, and money unburnt.
Keep It Cool, Keep It Dry
This compound draws moisture from the air like a sponge in a rainstorm. Storing it under dry, cool conditions stops it from clumping or breaking down into nasty byproducts. Humidity can start sneaking in through poorly sealed lids, and over time, the container’s weight increases, properties shift, and surprise reactions begin brewing. A dedicated dry cabinet, silica gel packs, and even a household dehumidifier can make a real difference.
Avoid the Light Show
Some chemicals go bad when they feel the sun’s rays or even just a bright bulb overhead. 1-Vinyl-3-Ethylimidazolium Bromide easily degrades in light, and that means you lose both money and performance. Use amber bottles or keep it tucked away in a closed, opaque cabinet. Store it near the back, away from windows and open bench lights. This prevents discoloration and breakdown, and it keeps the product’s reliability predictable for the next experiment or industrial run.
No Surprises, No Spills
Careless capping, cracked storage jars, or reusing old labels invite confusion and accidents. I’ve seen trainees grab the wrong bottle because of smeared writing, and one close call even meant an emergency room visit. Clear, up-to-date labels with the full chemical name and date of receipt go straight onto every bottle. Separate it from acids, bases, and oxidizers—cross-contamination always finds a way in chaotic cupboards. Lock up anything hazardous in a chemical storage cabinet that resists corrosion and keeps out wandering hands.
Check the MSDS—And Actually Read It
Shelf life depends not just on where a chemical sits, but also how often someone checks it. The Material Safety Data Sheet for 1-Vinyl-3-Ethylimidazolium Bromide gives storage details, recommended temperature ranges, and warnings for fire or reactivity hazards. This isn’t just a box to tick for compliance; it’s practical advice shaped by real-world incidents. I’ve seen horror stories about moisture causing a runaway reaction or improper storage jogging a sensitive compound loose during an earthquake.
Real Solutions—Not Just Rules
Talk with your safety officer or lab manager, and agree on a minimum standard for chemical storage. Install a temperature log if expensive stock is getting ruined. Rotate inventory so the oldest bottle gets used first, and schedule regular checks to catch leaks before they spread. Our lab built a habit of using QR codes for easy tracking, which means less time hunting for missing bottles and more time actually getting work done. Good practices are contagious—a tidy, well-organized shelf is one everyone wants to keep that way.
Responsibility Runs Deep
Safe storage of 1-Vinyl-3-Ethylimidazolium Bromide shows an organization values people, equipment, and outcomes. It leads to fewer surprises and better results in both research and industry. Proper care saves money, keeps people out of harm’s way, and builds trust across teams—all lessons my old lab learned the hard way, but ones I’m glad to pass along.
Understanding the Risks in Plain Terms
Working in the lab often brings a stack of unfamiliar bottles with labels long enough to twist the tongue. One chemical that pops up in research circles is 1-vinyl-3-ethylimidazolium bromide. It shows up in discussions about ionic liquids, green solvents, or specialty synthesis. The suit-and-tie names don’t make it less risky; in fact, they highlight how careful we all need to be. I remember my early days as a junior technician. I once thought new chemicals with few warnings meant less danger — it took a mild chemical burn to learn otherwise.
Let’s talk about 1-vinyl-3-ethylimidazolium bromide. This isn’t a household cleaner; it's no friend to bare skin. Ionic liquids have drawn plenty of praise for green chemistry, but plenty of research shows not every one of them lives up to the hype. Some versions, especially those with imidazolium cores, can cause trouble: irritation, skin dryness, even more severe toxicity if inhaled or injected. Lab tests from journals like Chemosphere and Green Chemistry tell a consistent story. The bromide versions can disturb cell membranes and, with concentrated exposure, trigger immune responses.
No Silver Bullet: Addressing Safe Practices
It’s easy to overlook the basics in the push for results. Gloves, goggles, and a sturdy fume hood sound like overkill—until a spill happens. One ill-placed drop on skin takes minutes to sting and hours to heal. The vapor is less noticeable than ammonia or bleach, but labs keep seeing cases of headaches, dizziness, or eye irritation after careless transfers. These symptoms usually fade, but repeated exposure builds up risk you can’t undo.
Some folks argue regulations run too conservative, slowing progress. My experience says a few extra minutes for glove changes or double-checking the MSDS beats missing a project day to see the occupational nurse.
Solutions Rooted in Reality
Training offers the real fix. Nobody picks up perfect lab habits from thin air. Supervisors carry the duty to hammer home good handling: never pipette by mouth, never leave containers uncapped, dilute spills right away, and keep hands away from face mid-experiment. Even minor details matter. Ventilation counts: carrying out syntheses only in well-ventilated hoods limits inhaled vapors, especially when the air stays dry and static-free.
Disposal takes another layer of caution. Many groups dump smaller samples down the drain, thinking volume turns danger into nothing. That’s shortsighted. Waste collection bins, labeled for halogenated organics, keep water treatment crews safer. Regular audits cut down surprise leaks or overfilled bottles, and direct lines of communication with university EHS teams make a huge difference.
Looking Ahead
The blend of curiosity and carefulness keeps research moving. More labs now use green chemistry checklists before ordering ionic liquids of any sort. New studies appear constantly on how to limit accidental exposure — some suggest swapping in less toxic salts, others focus on engineered glove coatings. Sharing lessons learned, in person and online, does more for safety than another shelf of unread binders ever could.
No chemical delivers innovation without some risk. Smart handling, informed decisions, and a low threshold for seeking help form a decent recipe for staying healthy in the lab. Based on what I’ve seen and the science backing it up, taking 1-vinyl-3-ethylimidazolium bromide lightly isn’t an option. Respect for the stuff — not just the formality of rules — makes all the difference.
The Realities Behind Purity Values
Tech jobs in chemistry keep pushing the limits on what’s possible, but sometimes the small details like purity ratings get overlooked in the big discussions. For a specialized ionic liquid like 1-vinyl-3-ethylimidazolium bromide, you don’t get far without thinking closely about the actual purity that gets shipped to researchers and manufacturing floors. Most suppliers label 98% as the minimum purity. This mark matters. Go lower, and trace contaminants can cause headaches ranging from reduced reaction efficiency to unpredictable results. Teaching labs and industry teams I’ve worked with often won't even test new ionic liquids that slide below this bar. It saves time and budget to start above 98% and avoid surprise batch failures.
Beyond that, I’ve noticed some suppliers offer “ultra pure” batches, hitting 99% or higher. Not every project calls for this level, but it makes a difference in sensitive applications like catalytic cycles or advanced battery test cells. Here’s the tradeoff: going for the highest purity might drive price up, stretch lead times, and shrink available quantities. Still, when working on anything where trace metals or organic residues would alter the course of your process, opting for premium quality pays off in the long run. I’ve seen multiple teams recover that upfront cost just by reducing troubleshooting down the line. This trade between cost and purity should always be part of the conversation, not just a footnote.
Practical Insights Into Packaging Sizes
Talk to anyone who orders chemicals for a lab or production line—the most common complaint isn’t just about purity, but about having to buy way more or way less than actually needed. Packages of 1-vinyl-3-ethylimidazolium bromide usually come in 5g, 10g, 25g, and 100g glass bottles. These increments make it easier to run pilot tests, scale up controlled reactions, or stock a few months of routine work without tying up cash in dead stock.
Companies that actually listen to customers will sometimes customize packaging for larger needs, jumping to 500g or 1kg drums on special order. The challenge hits small R&D groups especially hard, because buying a kilo just to use 50 grams means the unused chemical could degrade in the storeroom. I’ve watched more than one project stall out because of a mismatch between packaging sizes and experimental plans. Flexible, transparent packaging policies build loyalty—I look for suppliers that understand chemistry has real-world constraints.
Why Flexible Supply Chains Matter
Underestimating purity or misjudging packaging size can ruin experiments and eat-up budgets. One fix comes from suppliers who provide detailed Certificates of Analysis. These certificates let buyers know exactly what they’re getting in every bottle—a practice born from years of mistakes and process failures. Digital order systems, with real-time inventory tracking, have made life easier for everyone. Now, if I need something between 25g and 100g, many suppliers can split shipments or blend lots.
Transparency doesn’t just help end users either. It sets a signal across the sector: here’s a supplier who stands by their product, values traceability, and adapts to real lab needs. This open approach makes science more productive and supports safe, efficient progress. Strong sourcing and documentation practices now form a foundation for anyone using 1-vinyl-3-ethylimidazolium bromide and similar specialty chemicals.
Moving Forward With Informed Choices
Every scientist and engineer keeps a mental checklist—purity, reliable documentation, and packaging options always land near the top. Greater clarity on these points takes out some guesswork and lets researchers focus on breakthroughs, not supply chain irritations. A little extra investment in quality and logistics up front usually means smoother work, fewer delays, and stronger outcomes down the road.