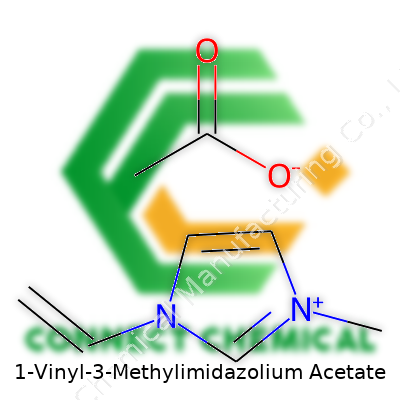
1-Vinyl-3-Methylimidazolium Acetate: A Deep Dive into Development, Properties, and Future
Historical Development
Looking back, the rise of ionic liquids like 1-vinyl-3-methylimidazolium acetate tracks closely with shifts in industry away from harsh solvents and outdated practices. Chemists in the late 20th century started experimenting beyond traditional salt and water solutions, searching for combinations that offer both chemical flexibility and a lower impact on the environment. The imidazolium core caught their attention: tough, adaptable, and stable, it welcomed a vinyl group and a methyl unit, all capped with an acetate for balance. This structure didn’t come out of blue-sky theory—it grew out of practical necessity, fueled by an increasing burden to reduce volatile organic compounds while maintaining effective chemistry in industries ranging from pharmaceuticals to materials engineering.
Product Overview
1-vinyl-3-methylimidazolium acetate goes far beyond the mean standard for ionic liquids. It’s a colorless to very pale yellow liquid, known for dissolving cellulose and other biopolymers. The core design, joining the imidazolium ring to a vinyl group, leaves the right kind of reactivity open. This molecular tinkering brings loads of researchers out of tedious trial-and-error work—giving them a ready way to manipulate viscosity, acidity, and compatibility with different reactants. Product lines offer bottles of various purities and packaging for industrial demand or lab scale, labeled for hazard awareness, batch tracking, and regulatory compliance.
Physical & Chemical Properties
In the world of ionic liquids, this compound holds its own for stability and solvating power. It has a melting point usually well below room temperature, a fact which lets it flow freely in most labs without special warming. The density tips a bit above water, around 1.1-1.2 g/cm³, offering enough heft for reliable mixing. Its thermal stability reaches upwards beyond 200°C, making it hard to decompose under standard processing. The acetate anion works hard: it grants miscibility with many polar and non-polar solvents and helps the molecule work as both a medium and participant in chemical reactions. High ionic conductivity makes this liquid a solid choice for electrochemical setups and certain battery applications. Its pH leans slightly basic compared to chloride alternatives, giving it unique effects when dissolving biopolymers.
Technical Specifications & Labeling
Technical sheets list purity often above 98%. Water content sits under 0.5%, since water can mess with reactivity. Most packaging comes marked with Chemical Abstracts Service (CAS) numbers, molecular formula (C8H13N2O2), and hazard statements—especially about skin and eye irritation. Labels also spell out the need for nitrile gloves, goggles, and fume hoods. Lot numbers, expiry dates, and supplier tracking codes help trace every batch from reactor to lab bench, a routine most chemical producers now strictly follow to keep risks low and reproducibility high.
Preparation Method
Getting to a bottle of 1-vinyl-3-methylimidazolium acetate from raw materials calls for a handful of careful steps. Most routes kick off with 1-methylimidazole, which gets alkylated by vinyl halides—usually vinyl chloride—under nitrogen or argon, producing the 1-vinyl-3-methylimidazolium intermediate. The vinyl function is touchy, so the reaction temperature, stirring, and inert atmosphere all must be kept just right to avoid unwanted polymerization. Following quaternization, a metathesis reaction introduces sodium acetate or another acetate source. The phase separation steps, along with careful washing, remove unreacted byproducts. Drying under reduced pressure strips out traces of water and volatile organics—not usually glamorous, but vital for handling and research.
Chemical Reactions & Modifications
Throwing 1-vinyl-3-methylimidazolium acetate into a reaction flask opens up plenty of options. As a solvent, it handles cellulose and other stubborn bio-based materials better than nearly anything else. The vinyl group, exposed and reactive, can take part in free-radical polymerizations. This lets chemists craft polymeric ionic liquids with custom properties, shifting viscosity or ionic strength by tweaking monomers. The acetate group brings nucleophilic character and can participate in reactions with epoxides, esters, and some organometallics. Its ability to dissolve a range of compounds means that it often acts as more than just a solvent, blurring lines between reaction medium and reactant.
Synonyms & Product Names
Depending on supplier or context, the compound goes by a few variations. Some call it 1-vinyl-3-methylimidazolium acetate, pairing the cation and anion for clarity. Others abbreviate it as [VMIM][OAc], reflecting the shorthand for organic ionic liquids. Brands might name it “VMIAC” for catalog purposes. Other names floating around include 1-vinyl-3-methylimidazolium ethanoate or simply vinylmethylimidazolium acetate. Regardless of label, the chemical identity doesn’t shift, but safety data sheets and technical bulletins remain essential reading for anyone planning to work with it.
Safety & Operational Standards
Lab safety can’t be left to luck. Though this ionic liquid won’t burn your nose like old chlorinated solvents, it can cause redness, burning, and tissue damage on contact. Nitrile gloves beat latex, goggles keep splashes out of eyes. Health and safety guidelines flag it for use only in well-ventilated areas, ideally under a chemical fume hood. Spills clean up with absorbent pads and proper disposal—not down the drain, not in standard trash. Companies and universities now treat ionic liquid waste as hazardous, following local chemical waste protocols. Training matters: reading MSDS forms, practicing emergency eye flushes, and wearing long coats all reduce the risk. Despite talk about “green” solvents, the word safe should never get thrown around without deep respect for the risks each bottle brings.
Application Area
Industries latch onto 1-vinyl-3-methylimidazolium acetate for what it can do—not what it promises. It stands out in biopolymer processing, especially dissolving cellulose to make films, fibers, and biodegradable plastics. Material scientists appreciate its role in forming conductive polymers, ion gels, and solid-state electrolytes for batteries. Research labs keep bottles ready for extracting bioactives from plants, synthesizing nanoparticles, or running reactions that traditional solvents can’t handle. Industrial players see promise in using it for recycling plastics, treating wastewater, and processing lignocellulosic biomass. Its chemical versatility reaches far—any process that needs tough solvents, high ionic mobility, or controllable reactivity gets a boost from this bench-stable ionic liquid.
Research & Development
A lot of grant money and late nights go into exploring ways to push the boundaries of what this compound can do. Research teams look at how subtle changes—switching the methyl position, tweaking the acetate group—shift performance for energy storage, catalysis, and even drug delivery. Journals carry stories of successes and setbacks. Cross-disciplinary teams link with engineers to scale up promising reactions or separate product from byproducts efficiently. Patent filings tick up as new uses for this ionic liquid emerge, from improved methods to dissolve stubborn plastics to optimized catalysts for green chemistry. Researchers gather in conferences, trading war stories and tips about cleaning up reactions, improving yields, and addressing bottlenecks in scale-up.
Toxicity Research
The toxicology work on 1-vinyl-3-methylimidazolium acetate isn’t just for regulatory red tape. Early findings show this compound sits at a lower hazard level than most traditional solvents, at least for acute exposure. Chronic toxicity still needs more work—bioaccumulation, environmental breakdown, and the impact on aquatic organisms sit high on the research list. Cell culture studies hint at some risk above certain levels, with membrane disruption and metabolic interference as possible issues. Animal studies continue, testing inhalation and dermal risks, but for now, clear ventilation, gloves, and prompt cleanup remain the frontline defenses. Researchers, regulators, and producers need to keep sharing new toxicology data—not so labs can check a box, but to prevent real-world harm years down the line.
Future Prospects
Looking forward, this ionic liquid stands out as both a challenge and a solution. More industries plan to move away from legacy solvents for processing renewables, plastics, and energy materials. With chemical and physical features that precision-tune for task and safety, 1-vinyl-3-methylimidazolium acetate arrives ready for next-gen batteries, new classes of polymers, and sustainable processing. Its biggest hurdles sit in scale-up cost, reusability, and full environmental fate. Developing improved recycling and recovery methods, tracking environmental breakdown products, and designing safer derivatives will drive the next wave of research. Students enter the field learning from last decade’s mistakes, pushing sustainability and performance beyond the hype. The future of this compound will depend on balancing the push for innovation with taking hard looks at health and safety, trusting no shortcuts, and staying honest about both success and failure.
Real-World Value in Green Chemistry
Chemistry continues pushing towards greener ways to get things done. 1-Vinyl-3-methylimidazolium acetate, often shortened to [VMIM][OAc], stands out as one of those chemicals that bridges old-school lab work and more eco-friendly processes. I’ve run into this ionic liquid in a few research settings, especially where researchers hope to ditch nasty solvents in favor of gentler options.
Its main claim to fame? Helping break down plant material—a process called biomass pretreatment. Ordinary solvents struggle to dissolve stubborn plant polymers like cellulose. 1-Vinyl-3-methylimidazolium acetate manages the job without producing toxic byproducts or releasing volatile fumes. That means safer working conditions and fewer headaches over compliance. Studies show it can convert raw corn stover or wood chips into useful sugars, setting the stage for biofuel production or biodegradable plastics. These are not just chemistry tricks; they matter when governments try reducing fossil fuel use.
Polymer Science and Innovation
Lab folks see value in its vinyl group, giving them a shortcut to polymerization. Long, chain-like molecules built using [VMIM][OAc] carry the ionic liquid’s unique traits: high thermal stability and good conductivity. The resulting polymers take center stage in specialized membranes—think fuel cells or batteries. In my time around energy storage projects, people look for membranes that let specific ions flow but block others. The acetate version of this ionic liquid forms films that last longer and handle heat better than materials used ten years ago.
Because it’s less flammable and less volatile than standard solvents, I saw it added straight to electrochemical setups. Researchers testing new batteries or supercapacitors want safer, more predictable lab results. Swapping out hazardous organic solvents for ionic alternatives like [VMIM][OAc] takes some worry off their plate.
Enzyme Reactions and Bioprocessing
Many researchers still rely on water for enzyme-catalyzed reactions. Yet water can hinder some bioreactions or cause unwanted side products. 1-Vinyl-3-methylimidazolium acetate creates a different reaction space, helping enzymes keep their structure intact. Sometimes the outcomes surprise people—enzymes produce better yields, even after being used multiple times. This kind of boost proves valuable for pharma firms, where each fraction of improved yield saves real money and energy.
Pushing for Real Change
Despite the hype, switching entire industries to new solvents doesn’t happen overnight. I’ve watched plants hesitate because of cost or lack of long-term studies. But academic papers continue to support [VMIM][OAc]’s lower toxicity, lower vapor pressure, and easy recyclability. These aren’t soft benefits; they feed right into new regulations and public pressure for cleaner production.
If labs and pilot plants team up with investors and policy teams, these greener solvents can move from small batches to the factory floor. Open-source data sharing helps speed up the process. My own experience? Highlight small wins first: safer handling, less equipment corrosion, easier waste disposal. Over time, people get more comfortable, and bigger changes start to stick.
The Question of Purity
Anyone working in synthetic chemistry runs across the question of purity—sometimes obsessively. 1-Vinyl-3-methylimidazolium acetate, used often in catalysis and as a solvent for cellulose, brings this conversation to the front. Synthesis rarely produces a single substance. Alongside the main product, side products, moisture, and solvents linger. Purity, in this context, tells you how much of your bottle actually gives you the chemical properties promised on the label, and not unwanted surprises during a reaction.
Practical Impact
The listed purity of 1-vinyl-3-methylimidazolium acetate usually falls above 97%, sometimes getting close to 99%. At first glance, those numbers feel high enough to brush off concerns. Real work reveals that even small impurities lead to stubborn inconsistencies. I’ve watched a reaction stall, only to discover trace chloroacetate snuck in from a precursor. Moisture absorption often causes problems too; a hygroscopic salt like this pulls water straight out of humid air.
What “Pure” Really Means
A reagent might claim 99% purity. This number often skips over unidentified side products and doesn’t account for breakdown during storage. For ionic liquids like this imidazolium derivative, the acetate anion sometimes degrades, especially when the bottle sits on the bench for long. Impurities tend to sneak in during scale-up. What worked for a 20-gram lab sample might surprise you in a kilogram batch, or when you switch suppliers. Certificates of analysis help, but unless you check by NMR or HPLC yourself, you rely on vendor trust. I’ve seen colleagues run a simple proton NMR, only to discover the “impure” spectrum reads like a complicated mystery novel.
Why Should We Care?
A project almost always goes more smoothly when chemicals behave predictably. In catalysis or materials science, trace metal contaminants strip away the magic. Acetate impurities sometimes lead to odd polymerization or cause cellulose to dissolve poorly. Some labs accept this, run pilot tests, and see if performance tanks. Others, dealing with regulatory paperwork or pharmaceutical applications, can’t afford this risk. Purity takes on more significance in these regulated industries, where the presence of residual solvents or excess cations can derail months of development and draw the wrong kind of auditor attention.
What Solutions Actually Work?
I’ve found the fastest way to avoid headaches is to buy small quantities, check the certificate, and test new lots by NMR or other simple analyses. Some labs take an extra step and distill or recrystallize the salt—tedious, but effective. Others reach out to vendors for batch-specific data and keep tight records. Securing a reliable supplier can sometimes matter more than wrangling with purification yourself, but switching suppliers mid-project brings its own headaches. I’d suggest storing the salt under dry nitrogen and using a desiccator, since this material likes to collect water whenever it can.
Weighing Real Risks
1-vinyl-3-methylimidazolium acetate brings a lot to the table as a solvent and catalyst. Its utility depends on an unspoken agreement: that purity means what the label says. Small-scale chemistry can tolerate some variance, but for anything critical, running that extra purity check, staying in good communication with vendors, and recording any odd results saves hassle. This might mean holding off on bulk orders until the first batch clears. While rare, changes in vendor process or packaging sometimes alter quality, so keeping tabs on every step matters. Watching out for impurities or breakdown keeps your science honest, and that benefits everyone down the line.
Understanding the Substance
Anyone who’s ever handled chemicals in a lab knows storage can make or break both safety and results. Take 1-Vinyl-3-Methylimidazolium Acetate, for example. This ionic liquid pops up in a lot of research settings, and its storage often gets overlooked. I’ve spent some time in shared academic labs, where basic principles matter just as much as the complicated science. Simple mistakes—like leaving a container loose or letting moisture creep in—can wreck expensive samples or lead to nasty surprises on your next shift.
The Basics of Safe Storage
This chemical shows a keen sensitivity to moisture and air. In my experience, exposure to humidity starts a chain reaction: clumpy material, reduced purity, unpredictable behavior in experiments. Manufacturers list its shelf life on the assumption it stays sealed tight and cool. I keep it shut in an airtight glass bottle, usually with a fresh desiccant sat nearby. Plastic containers sometimes change shape or leach over time, especially if solvents or acids stand nearby—glass saves a lot of headaches.
Chilled but Not Frozen
Cool storage gives a big advantage. Most protocols recommend conditions around 2–8°C, like a standard laboratory refrigerator. I’ve tried skimping and sticking things on a shelf at room temperature for convenience, only to find residues at the bottom of the bottle later. There’s a temptation to push bottles into a freezer for “extra” protection, but 1-Vinyl-3-Methylimidazolium Acetate turns sluggish when frozen, making it frustrating to work with after thawing. Fridge temperature delivers stability without the hassle.
Keep it Dark, Keep it Clean
Light can play tricks on sensitive liquids. Direct sunlight through a window, or even a cracked fixture, degrades imidazolium-based compounds with surprising speed. I stash bottles in opaque secondary containers or tuck them in the darker corners of storage units. Dust collects on labels and lids in a shared lab, so a quick wipe each time goes a long way to prevent grit from contaminating the sample. Clean hands need to be a habit—some of the worst spills I’ve seen started with a single careless touch.
Labeling and Tracking
Proper labeling might sound basic, but confusion pops up fast in a busy space. I always use chemical-resistant markers, add the date opened, and include hazard details. Keeping a log—manual or digital—means anyone can check status or shelf life at a glance. Inconsistent labeling once led to a close call at my old lab, where someone mistook the compound for a less hazardous salt.
Community Habits and Solutions
Solving gaps in chemical management often starts with habits, not just rules. Assigning clear roles for checking expiration dates, running regular cleanups, and sharing near-miss stories helped boost safety in my experience. As new chemicals enter the market, the same discipline pays off. Down-to-earth communication beats a dusty manual every time. With a careful approach, labs cut waste, avoid health risks, and get the results they actually want from these modern materials.
Why Lab Safety Matters More Than a Checklist
Lab work brings a natural rush, especially when opening a new bottle of something with a long, complicated name. My early days in research taught me how even small mistakes around chemicals like 1-vinyl-3-methylimidazolium acetate can trigger chaos from an accidental splash. Stories stick with you—someone gets careless and leaves a glove out, suddenly there's a stinging hand or a spill on a notebook. These aren’t the kind of lessons a person forgets.
Understanding the Risks
1-Vinyl-3-methylimidazolium acetate works as an ionic liquid, used often for dissolving cellulose or as a reaction medium. The structure makes it good at breaking things down, but this isn’t so friendly when it touches your skin or eyes. Research journals describe it as corrosive to skin and eyes. That means redness, burns, or long-term damage after only a short exposure. Breathing vapors isn’t a great idea either. Even if the smell doesn't hit you, some of these chemicals cause respiratory issues unnoticed until later on.
Solid Precautions for Handling
Real safety starts before anyone uncaps a bottle. Set up every reaction in a fume hood to trap fumes and block accidental splashes from spreading. Even a small mishap lands inside the hood’s range. Nobody wants that stuff wafting out across the benches.
Personal protection means more than a lab coat. Pull on goggles that hug close to your face. Shield your hands with thick nitrile gloves; latex tends to break down faster around solvents and ionic liquids. Use a face shield if there’s a genuine risk of splashing. Remember to tie back hair and keep sleeves tight so nothing drags through the liquid.
Proper Storage and Cleanup
Every bottle goes in a ventilated chemical cabinet. Don’t stash acids, bases, or oxidizers near it. These ionic liquids sometimes break down or react with other groups. Bottles should sit upright, sealed tightly to block leaks.
Spills make the heart jump, but fast action limits the damage. Cover the spill with a solid absorbent, sweep it into a waste container, and skip the urge to wipe with just a paper towel. If any touches skin, wash right away with cool water and soap—don’t wait, don’t shrug off even a prickly feeling.
Why Training Always Beats Guessing
The main problem comes when new staff skip safety training or assume instructions don’t apply. I’ve watched a researcher rush, thinking they’re immune to hazard since nothing happened the last time. Real experience says otherwise: anything dangerous finds a shortcut past overconfidence. Better to drill everyone—old hands and newcomers—on real emergency procedures. Show exactly where to find eyewash stations and safety showers. Don’t just point them out on a map.
Responsible Waste Disposal
Leftover material shouldn’t end up in the sink. Collect it in a labeled waste container and send it through the proper disposal channels. Improper disposal threatens both water systems and the people handling waste downstream. Following waste protocols means no surprises for janitorial or maintenance crews.
Everyone’s Role Matters
Anyone working with 1-vinyl-3-methylimidazolium acetate shares the responsibility to protect themselves and those nearby. Slipups impact more than just the person holding the pipette. With some planning, respect for the risks, and the right gear, labs run smoother and nobody deals with avoidable pain.
Looking Beyond the Product Catalog
People asking about bulk availability of 1-Vinyl-3-Methylimidazolium Acetate usually need it for research, pilot projects, or early-stage scale-up. Chemical suppliers know this, but not every specialty material sits in warehouse drums waiting for a phone call. Take it from anyone who’s worked in a lab or managed a small process scale-up: you’ll run into roadblocks between the catalog and the actual shipping dock.
Many suppliers list this compound online, but these offers typically top out at a few hundred grams. That serves most academic groups or method development. Phone calls or email exchanges reveal a different story for anyone who wants kilos or more. I learned quickly that availability doesn’t always mean fast delivery or regular supply. Some of these companies only make a batch after someone places an order—and please expect to pay upfront. It’s never as simple as clicking “add to cart” and getting a commodity shipment.
The Backbone of Ionic Liquid Markets
As ionic liquids began showing up in green chemistry, battery work, and cellulose processing, more groups sought reliable bulk sources. Companies stepped in, but their focus stayed close to demand. 1-Vinyl-3-Methylimidazolium Acetate entered routines as a cellulose solvent or reaction medium thanks to its high thermal stability and solvating behavior. Its vinyl group turned it into a candidate for polymer applications too. Analysts at Frost & Sullivan or MarketsandMarkets have pointed to ionic liquid growth rates in the range of 8-12% over the next decade. These are not commodity numbers but indicate scaling interest.
Global producers—Alfa Aesar, Iolitec, Synthon Chemicals, Merck—offer this compound but rarely stock large drums. Some niche suppliers in China and India will scale up synthesis on request, pulling from stored precursors and tweaking their reactors. Buyers will need to negotiate delivery timelines, purity guarantees, and pricing. This creates a spot market, where timing and logistics shape the deal.
Quality, Logistics, and Hard Numbers
Sourcing specialty chemicals in sizable lots has always brought headaches, whether it's customs paperwork, purity documentation, or freight clearances. During a past reactor cleaning project, a shipping delay of just 72 hours cost thousands in downtime. Peroxide-sensitive or hygroscopic liquids like 1-Vinyl-3-Methylimidazolium Acetate demand temperature monitoring and special containers. Analysts at Grand View Research flag that handling and supply chain complexity add to overall cost by 10-25% for many ionic liquids.
Every buyer should ask for measured purity, moisture analysis, and synthetic route details. Some suppliers cut corners with recycled solvents or generic acetate, which leads to erratic performance. Industrial users should insist on batch testing, and ideally, keep a backup supplier on file.
Workarounds and Solutions
Cooperative purchasing groups, long-term agreements, or direct engagement with producers make things easier. One large European university built stable supply by promising to buy multiple related compounds, letting the supplier optimize one production run. Some US-based startups partnered with local chemical distributors who handled import certifications and ensured compliance. For buyers tackling sustainability goals, discussions about lab waste, recycling, or green sourcing are already reshaping specifications.
Industry connections, honest communication, and persistence open doors. People persistent enough to phone around, share details of end-use, and negotiate realistic lead times usually get what they need. Expect some legwork—but workable options do exist for anyone committed to their process and willing to adapt.