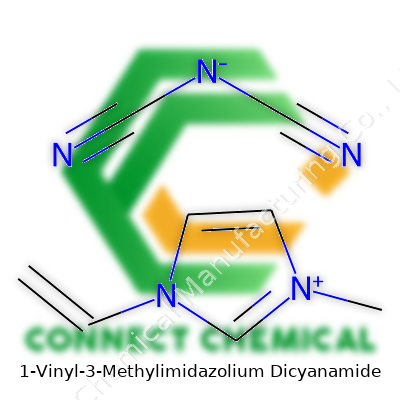
1-Vinyl-3-Methylimidazolium Dicyanamide: A Real-World Look
Historical Development
Chemists started exploring ionic liquids a few decades back, chasing the dream of cleaner, more versatile industrial solvents. Among these, 1-vinyl-3-methylimidazolium dicyanamide carved out a spot thanks to its unique stability and reactivity. Its roots trace to groups looking for compounds that could handle demanding reactions without catching fire or mixing with water every time. Early work often centered on simple imidazolium salts, but as the push for “greener” chemistry caught fire, this compound drew serious attention for both lab-scale experiments and emerging industrial processes. My own experience in university labs saw this compound pop up again and again as a solution where tradition solvents made a mess.
Product Overview
1-Vinyl-3-methylimidazolium dicyanamide combines a robust imidazolium ring—famous for its resilience—with a dicyanamide anion, which brings flexibility to the chemistry table. In the bottle, it looks like a colorless or pale yellow liquid and doesn’t give off much of an odor. People working with this compound say that it handles heat and pressure better than a lot of classic organic solvents, so it’s found its way into labs trying to cut down on waste and scale up reaction yields. Its resistance to hydrolysis stands out, allowing for repeated use in tough environments, which saves both effort and cost.
Physical & Chemical Properties
Ask any chemist who’s handled 1-vinyl-3-methylimidazolium dicyanamide, and they’ll mention its low melting point and high solubility. This ionic liquid usually melts somewhere below 100°C and has strong ionic conductivity, important for electrochemical setups. Density tends to fall between 1.1 and 1.2 g/cm3, and it remains stable across a wide pH range. Its viscosity can get tricky, especially at room temperature, but warming it up smooths out handling. Despite being salt-based, it avoids crystallizing at lower temperatures, so you can pour, measure, and transfer without much fuss.
Technical Specifications & Labeling
Reliable suppliers of this compound label every drum or bottle with batch number, purity—often boasting 97% or higher—water content, and hazardous material icons, since it requires careful handling. Companies that take safety seriously will add storage recommendations, best-before dates, and a safety data sheet. When I worked in industry, the focus usually sat on avoiding moisture buildup and making sure all containers had tight seals, as dicyanamide-based compounds sometimes pick up small amounts of water, which can mess with sensitive reactions.
Preparation Method
Most production flows by reacting 1-vinyl-3-methylimidazolium chloride with sodium dicyanamide in solution, then filtering off the sodium chloride byproduct. The wash and evaporation steps can’t be skipped, or you end up with impurities that tank the yield and complicate downstream reactions. Teams looking for pure product tend to run extra washes or use vacuum drying. Purity matters here—every percent counts when scaling up to bigger batches because even tiny contaminants show up in the final performance.
Chemical Reactions & Modifications
This ionic liquid steps in as both a solvent and catalyst in organic synthesis, especially for alkylation, coupling, and polymerization. Chemists tweak the imidazolium ring by swapping out substituents, giving the molecule more bite or flexibility based on what needs to get done. Dicyanamide’s resonance properties open the door to coordination chemistry, letting this compound stabilize metals for catalytic cycles. It interacts with strong acids or bases, but what sticks with me is how the nitrogen backbone resists degradation even in tough conditions, opening up new reaction channels unavailable to older, more brittle solvents.
Synonyms & Product Names
In catalogs and studies, you might spot 1-vinyl-3-methylimidazolium dicyanamide under names like [VMIM][DCA], 1-vinyl-3-methylimidazolium dicyanamide ionic liquid, or just VMIM DCA. Some supply houses drop the long chemical name in favor of quick codes, but it’s all talking about the same core structure, with small differences in cation or anion when shifting to related salts. Double-checking CAS numbers makes life easier, especially with similar imidazolium compounds thrown in the mix.
Safety & Operational Standards
This isn't a chemical to mess around with lightly. Spill it on your skin, and irritation fires up right away. Ventilation stands top priority during handling, since some byproducts can volatilize under heat. Standard procedures involve gloves, goggles, and working under a hood. Waste disposal calls for neutralization—not just pouring it down the drain. I’ve seen smart labs set up dedicated waste streams because ionic liquids, even the “safer” ones, raise flags on possible waterway contamination due to their persistence and unknown breakdown routes.
Application Area
Electrochemistry labs count on this compound for its stability in batteries, sensors, and fuel cells. Polymer chemists harness its solvent power to drive tough syntheses and yield new materials, especially in controlled radical polymerizations. In extraction technologies, it shows up separating metals or bio-compounds, taking pressure off older solvents that pollute or degrade easily. Teams working on CO2 capture and catalysis also lean on this compound’s tough chemistry, reducing waste and cost in industrial pipelines. My own work involved purifying specialty markers in medical testing, where only highly stable ionic liquids did the trick.
Research & Development
Universities and corporate labs run studies to boost performance in battery electrolytes, tweak viscosity, and expand its scope in organic transformations. Some push for lowering production costs—right now, the price of pure ionic liquids still slows down widespread adoption. Scientists tinker with different cation and anion swaps, searching for combinations that outperform the old guard in terms of efficiency and sustainability. In R&D meetings, cost and supply chain headaches come up a lot, but the unmatched reactivity still draws researchers back for more testing.
Toxicity Research
Toxicity gets complicated. While ionic liquids once wore a “green” label, recent studies highlight their persistence in soil and water, raising concerns over chronic exposure. Acute toxicity studies show mild skin and eye irritation, though longer-term effects need more exploration before anyone can call them truly safe. My contacts in regulatory affairs stress rigorous monitoring—not just for workplace exposure, but for manufacturing emissions and lab waste streams. Teams value fast-acting spill responses and updated MSDS sheets, pushing for transparent risk assessment in every stage between synthesis and disposal.
Future Prospects
The future for 1-vinyl-3-methylimidazolium dicyanamide turns on the research race to build greener, safer solvents. As traditional industries look to phase out toxic or volatile organics, this compound could step in, given enough cost reduction. More teams in battery and materials science aim to fine-tune its properties, searching for the sweet spot between performance and environmental footprint. While hurdles around mass production, toxicity, and recyclability need answers, growing pressure for sustainable manufacturing is likely to spark fresh investment and innovation in this class of compounds. Watching the next five years, I expect to see more tailored ionic liquids finding roles in pharmaceuticals, green chemistry, and high-tech manufacturing, as the balance between safety, performance, and affordability sharpens with experience and feedback from both industry and research.
More Than Just a Complicated Name
1-Vinyl-3-Methylimidazolium Dicyanamide shows up in labs and workshops where precision chemistry plays a key role. This room-temperature ionic liquid may sound like a mouthful, but in practice, it makes serious contributions to energy research, environmentally-minded manufacturing, and chemical synthesis. It doesn’t serve as just another fancy solvent. It’s valued for specific qualities—thermal stability, low volatility, and its ability to dissolve tough customers like cellulose and certain metals.
What Makes This Compound Stand Out?
Traditional solvents like toluene or benzene bring baggage, including high volatility and dangerous fumes. 1-Vinyl-3-Methylimidazolium Dicyanamide stands apart because it doesn’t evaporate easily and resists breaking down when things heat up. It’s also non-flammable, and its structure can get tweaked for a range of purposes. These seemingly small advantages become critical with a growing demand for greener, safer lab and industrial processes.
Energy Storage and the Push for Better Batteries
Researchers in the battery space pay attention to how ionic liquids behave as electrolytes. Lithium-ion and sodium-ion battery projects often test dozens of alternatives, hoping for improved capacity and longer life. This dicyanamide-based compound shows strong conductivity, which helps ions move faster and batteries charge better without catching fire or degrading. While it’s not yet at every hardware store, people who spend time with battery cells in the lab have tested and published on it time and again. Improvements in safety and storage capacity will matter more as more cars and homes rely on rechargeable power sources.
Eco-friendlier Alternatives for Production
A lot of synthesis in both research and industry focuses on minimizing hazards. 1-Vinyl-3-Methylimidazolium Dicyanamide draws interest because of lower toxicity and its eager recycling behavior. In processes such as cellulose dissolution—think textiles and biomass upcycling—it breaks down plant matter that otherwise resists common solvents. The world needs better, less-polluting ways to turn waste into value, and ionic liquids like this one help open those doors.
Catalysis and Material Science
Not every reaction goes smoothly with classic solvents or traditional catalysts. This compound often plays a supporting role in catalytic cycles, where chemists want selectivity or need to stabilize transition metals. Its imidazolium center and dicyanamide anion interact in ways regular organic solvents can’t match. I’ve seen paper after paper reporting how these properties lead to cleaner conversions and easier product separations. A few industrial players have started using it to polish surfaces, strip coatings, or even help with making nanomaterials.
Pitfalls and What’s on the Horizon
Packing all these strengths doesn’t mean this ionic liquid fits everywhere. Cost sits fairly high compared to everyday chemicals. Large-scale manufacturing also asks tough questions about lifecycle impact—just swapping out one substance for another doesn’t always mean a greener process. Regulators and research teams keep pushing for fuller profiles: what breaks down, what lingers, and what makes it into water streams? More direct answers build trust and make these materials reliable for a growing set of industries.
Room for Progress and Solutions
To broaden its use, lab teams need reliable ways to produce it from accessible chemicals and recycle after use. Regular monitoring and transparent reporting help address concerns before they scale. University groups, private labs, and big business all have a role. Investment in data-sharing and best-practice guides could help the field mature faster, and that means better batteries, smarter recycling, and safer labs for everyone.
Getting Real About Chemical Risks
If you work in labs or manufacturing, unfamiliar chemical names probably pop up all the time. 1-Vinyl-3-methylimidazolium dicyanamide sounds technical, but the concerns around it feel pretty familiar to anyone who’s spent time with industrial chemicals. I’ve handled countless bottles over years as a researcher. Reading labels carefully, watching safety sheets, and keeping one eye on the news about chemical incidents—these become second nature. The real question is: Does this substance deserve extra respect for toxicity or hazard?
Known Facts about the Compound
1-Vinyl-3-methylimidazolium dicyanamide falls into the category of ionic liquids. These compounds started getting attention for replacing solvents that pollute or cause health problems. Compared to older, more volatile solvents, ionic liquids tend to act more stable and stick around longer. The upside: less air pollution and fire risk. There’s a flipside too. That lasting power can put strains on waste streams and clean-up protocols if things go sideways. Some ionic liquids end up more persistent, and there’s mounting research showing not all of them are as green as early headlines promised.
What Science Says about Toxicity
Early tests on 1-vinyl-3-methylimidazolium dicyanamide showed low volatility, so breathing in dangerous fumes seems less of a risk compared to classic lab solvents. Reports from the past decade flag moderate toxicity toward aquatic life. In the lab, accidental exposure—skin or eye contact—can cause irritation. The dicyanamide anion introduces new complexity, as some related compounds break down and release small amounts of cyanide under certain conditions. That’s serious. At high enough levels or with long exposure, real risk exists, especially for water systems and people working up close with the raw material.
Why Regulation and Vigilance Matter
I learned the hard way that lack of data equals risk for workers. Many newer chemicals don’t show up in long-term studies, so it becomes important to treat every new compound like it could have downsides we haven’t seen yet—especially in bulk use. Major chemical safety agencies in the US and Europe have flagged data gaps for many ionic liquids, including this one. So far, there’s no sweeping ban, but major users like academic labs and chemical plants put workplace exposure controls in place: gloves, splash goggles, solid ventilation, and sealed containers. Waste disposal matters too, because this stuff in the water table could be rough for fish and microbes. Trade associations keep suggesting detailed environmental impact studies before ramping up use too much.
Steps for Better Handling
No one wants to see a repeat of the slip-ups that happened with now-banned chlorinated solvents. Anyone handling 1-vinyl-3-methylimidazolium dicyanamide can take simple steps to cut danger: stick with closed systems, label everything in plain language, collect waste carefully, and run regular air and water checks nearby. Chemical companies get tasked with digging deeper into long-term health risks. Regulators have a role, but responsibility also lands on companies, lab heads, and individual researchers to push for transparency and safer alternatives if the science shows warning signs.
Looking Ahead
It’s tempting to hope for miracle green chemicals, but every replacement brings new hazards. Experience shows that skepticism and active hazard controls usually pay off. Instead of trusting buzzword claims, asking tough questions about toxicity and environmental footprint—before ramping up production—keeps health and safety up front. These conversations don’t slow down science; they keep us from repeating old mistakes, especially when it comes to substances as persistent and unpredictable as this one.
Practical Steps for Safe Handling
Some chemicals demand respect before they even enter the lab. 1-Vinyl-3-Methylimidazolium Dicyanamide falls in that group. My own time working behind the bench has taught me that small misunderstandings with hygroscopic chemicals or reactive salts cause major headaches—or worse, accidents. This isn’t just another “keep away from sunlight” routine, since ionic liquids such as this one test the patience and preparation of even seasoned chemists.
First off, moisture control takes top priority. Dicyanamide salts love water, and they go out of their way to pull humidity out of thin air. A tightly sealed, air-tight storage container keeps the contents from clumping up or breaking down. Sticking a fresh silica gel pack or another desiccant in the cabinet acts as backup. Here’s something I’ve seen: trust plastic bottles with gas-tight lids over glass with stoppers that can wick moisture inside. Shoving the bottle deep in a desiccator rarely lets you down, especially in muggy climates or crowded storerooms.
Temperature and Light Control
Heat speeds up the chance for chemical changes you didn’t sign up for. 1-Vinyl-3-Methylimidazolium Dicyanamide won’t catch fire on a sunny windowsill, but all sorts of side reactions get a leg up if temperatures wander above room temperature. A cool, dry shelf below 25 °C in a shady corner fits the bill. Constant temperature keeps the shelf life predictable—no random crystallization or breakdown turning a clear liquid into a mess overnight. I’ve boxed up enough degraded samples over the years to learn that a simple thermometer taped to the cabinet door saves hundreds of dollars.
Direct sunlight can quietly ruin more than just old photographs. Ultraviolet radiation changes organic salts on a molecular level. Storing the compound in brown or opaque containers helps block stray light. Stash containers at the back of a cabinet or in a box with a lid—nobody will ever regret keeping this kind of chemical just out of sight.
Avoiding Common Contaminants
Purity matters, especially when your research depends on every decimal. Dicyanamide picks up dust, traces of organic materials, and other solvents much faster than you think. Regularly wipe down benches and labels, check for leaky lids, and only use dry, clean scoops or spatulas for transfers. Skipping these simple steps opens the door to impurities, which give false readings when it counts most.
Label Everything—Double Check Every Time
Experience tells me a mislabeled bottle finds a way to cause problems. Name, date received, and hazard info should pop out at a glance. I encourage coworkers to use bright hazard stickers, even if regulations only demand a small symbol. Double-checking before opening anything avoids surprise reactions or cross-contamination. Safe storage starts with clear communication—never just a good habit, always a non-negotiable.
Emergency Prep Isn’t Optional
Accidents threaten more than loss of expensive chemicals. Asphyxiation or chemical burns drift into the picture with the wrong handling. Emergency eyewash and spill kits belong within reach. Sharing protocols during training and keeping up-to-date safety data sheets near storage areas protects both people and research outcomes.
Safe, thoughtful storage keeps research moving and people safe—every day, every bottle.Understanding the Compound
1-Vinyl-3-methylimidazolium dicyanamide feels like a string of syllables, but tucked inside, there’s a story that matters to materials science, chemistry labs, and green technology. Start with the formula: its cation, the 1-vinyl-3-methylimidazolium, carries C6H9N2+, and the anion, dicyanamide, is N(CN)2- or C2N3-. Put them together, and you get C7H9N5.
Relevance in Chemistry and Industry
The rise of ionic liquids has turned some attention to molecules like this. Ionic liquids usually melt around room temperature and can stay stable when exposed to air or heat. That means less worry about equipment corrosion and hazardous vapors filling the air in research or industry spaces. I’ve seen researchers gravitate toward tools that cut down on risks and improve results, and this ionic liquid fits that need well.
Dicyanamide-based ionic liquids often stand out for use in electrochemistry, catalysis, and even batteries, because they conduct ions efficiently and offer low volatility. Chemists working to replace traditional solvents (that often carry health or safety baggage) look for safer and more flexible compounds. 1-Vinyl-3-methylimidazolium dicyanamide provides a stable platform. It’s less flammable than traditional organic solvents, allowing for safer workspace conditions. The vinyl group offers routes for polymerization or functionalization, creating polymeric ionic liquids or new hybrid materials with custom properties.
Solving Real Problems
Waste and toxicity plague conventional solvents. My own experience handling organic chemicals brings home how routine practices used to rely on volatile substances, which could evaporate or leak toxins. Ionic liquids like this one bring in the possibility of closed-loop uses — difficult to reach with older chemical salts or liquids. Their negligible vapor pressure means less inhalation risk and no ugly chemical smells. That’s not just a comfort issue; it makes compliance with environmental and workplace safety standards easier to achieve.
Growth in energy storage and sustainable catalysis leans on molecular innovation. With a formula like C7H9N5, combining the imidazolium ring (known for its ionic stability) with the dicyanamide anion (recognized for coordination and electrical conductivity), you get a blend that serves as a testbed for battery electrolytes and advanced sensors. There’s movement toward using such chemicals in resource recovery, such as extracting valuable metals from e-waste or catalyzing difficult organic reactions with high selectivity and low environmental penalty.
Facing the Trade-Offs and Moving Forward
Every new material brings trade-offs. Synthesis costs and scalability come to mind right away. I’ve known colleagues to weigh the price of specialty chemicals against the benefits: will the ionic liquid hold up over dozens of reaction cycles, or end up as another lab curiosity with limited use? Life-cycle analysis should shape decisions, from raw material sourcing to final disposal or recycling options. Researchers and manufacturers have begun to network more, sharing best practices, and leaning on published safety and performance data. Optimizing these ionic liquids for industrial processes remains a challenge worth tackling, aiming for a better balance between innovation, sustainability, and economic feasibility.
An Everyday Question with Far-Reaching Impact
People rarely talk about 1-vinyl-3-methylimidazolium dicyanamide at the dinner table. For those who work with chemicals, though, its water solubility isn’t just trivia—it’s a doorway to everything from greener chemistry labs to cleaner energy systems. Solubility sounds simple. You put a substance in water. Does it mix, or does it clump in the corner? This is a chemical with a mouthful of a name that answers by sliding into solution with water, showing off the power and strange charm of ionic liquids.
The Science Is Clear
This dicyanamide salt—a member of the imidazolium ionic liquids family—has drawn plenty of eyes for its mix of organic structure and unusual anion. Unlike some ionic compounds that sit on the bench like stubborn grains, 1-vinyl-3-methylimidazolium dicyanamide dissolves in water with ease. Labs across Europe, Asia, and North America have reported that this compound forms clear, homogeneous solutions even at room temperature. It owes this trick to the balance between its polar components and that dicyanamide group, which breaks up water’s hydrogen-bonded network and invites the ions in.
Chemists, including myself back in grad school, love working with materials that readily get along with water. It means we can skip strong organic solvents that make safety goggles fog up and heads ache. Water as a solvent also runs cheaper and feels better for the environment compared to something like acetonitrile. A journal from the Royal Society of Chemistry described imidazolium-based salts such as this one behaving as “hydrophilic” and able to pull off efficient dissolution without elaborate equipment.
Why Care About Solubility?
Solubility brings a stack of practical uses. In the field of catalysis, water-soluble ionic liquids pave the way for reactions that waste less and pollute less. The vinyl group in this molecule opens doors to polymerization right in water, side-stepping the need for harsh chemicals. Green chemistry journals have highlighted that one key to more sustainable manufacturing sits in swapping out persistent organics and using water instead. If a molecular designer dreams up a new ionic liquid, high water solubility makes it easier to scale, manipulate, and recover.
Electrochemists rely on these ionic liquids to boost conductivity in water-based batteries and supercapacitors. I met a startup founder at a conference who swore by dicyanamide solutions for their controlled conductivity and low viscosity, as they tried to build safer, non-flammable batteries for off-grid energy storage. In biochemical analytics, this solubility trait makes wide-ranging extraction processes possible, especially in separating metal ions from waste streams or tweaking enzyme activity in research settings.
Challenges and Looking Forward
It’s not all blue skies with these salts. High solubility can create headaches—imagine contaminant removal from industrial wastewater if such chemicals escape without proper containment. Monitoring, regulation, and better engineering controls sit right at the top of any responsible use list. Environmental groups keep a close watch on how these compounds break down. Current research points toward slow degradation for some ionic liquids, so industry and science can’t afford to overlook long-term waterway impacts.
Getting a grip on safe handling, better discharge controls, and smarter lifecycle analysis will shape how these compounds play roles outside the lab. Green chemistry teaches that every breakthrough brings new stewardship duties. Soluble or not, every molecule needs to pull its weight for a cleaner, safer, and more sustainable future.