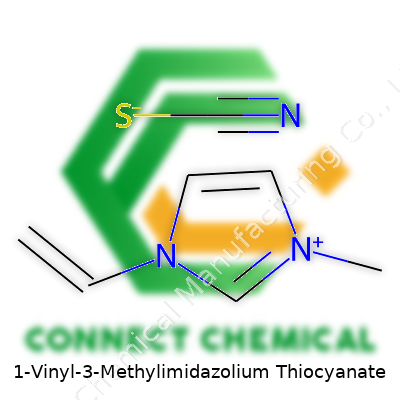
1-Vinyl-3-Methylimidazolium Thiocyanate: A Deep Dive
Historical Development
Chemists started looking at imidazolium-based compounds in the late twentieth century with fresh eyes, especially as the green chemistry movement gained traction. I remember discussions during my grad school days, folks pinning up papers about room-temperature ionic liquids and how these salts could change the way we think about solvents and electrolytes. The story of 1-vinyl-3-methylimidazolium thiocyanate tracks back to this era, emerging as part of a search for safer, more customizable ionic compounds. As the field pushed past basic imidazolium chlorides, labs explored pairing the imidazolium cation with less-studied anions like thiocyanate, seeking better solubility and unique electrochemical properties for applications that weren't possible with traditional salts or solvents.
Product Overview
What sets 1-vinyl-3-methylimidazolium thiocyanate apart is that vinyl group dangling off the imidazolium ring. Most ionic liquids focus on stability and non-volatility, but throw in that vinyl, and you’re talking about a molecule that can also take part in polymerization. I've seen research teams use this property to produce conductive polymer films—materials combining the chemical resilience of ionic liquids with the mechanical properties and processability of plastics. This gives manufacturers a wider toolset, not just for lab-scale experiments, but for scalable solutions in antistatic coatings, electrochemical devices, and specialty materials.
Physical & Chemical Properties
1-vinyl-3-methylimidazolium thiocyanate, like many ionic liquids, resists evaporation and remains liquid at room temperature. I kept a bottle on my shelf for months, and it barely lost a drop. The compound's color usually falls between pale yellow and faint amber, hinting at its chemical activity. You notice a faint odor, somewhat reminiscent of mustard or garlic, likely from the thiocyanate anion. It dissolves in water and many polar solvents, enabling straightforward mixing and application. The density comes in above most organic solvents, while viscosity can vary based on temperature and small changes in impurity levels—something that always made cleaning glassware a bigger chore than with standard organics.
Technical Specifications & Labeling
Product labels for 1-vinyl-3-methylimidazolium thiocyanate need more than a casual glance. Purity usually sits at or above 98%, but trace amounts of parent imidazole or residual halides reveal details about the synthesis quality. In my experience, reputable suppliers include precise water content, since ionic liquids soak up moisture from the air. You also want info on residual solvents, conductivity standards (typically measured in S/cm), and free acid content. Each batch needs a Lot Number for tracking, and you'd better see hazard codes and storage advice printed clearly, especially since the thiocyanate anion carries health and environmental warnings.
Preparation Method
The route to 1-vinyl-3-methylimidazolium thiocyanate usually takes two or three steps. Starting with N-methylimidazole, chemists alkylate it with vinyl halides—this part always needs dry, oxygen-free conditions, or yields nosedive. After isolation of the vinylated product, they swap out the original anion (usually chloride or bromide) via simple ion exchange with potassium thiocyanate in water or methanol. Every time the color or odor drifted during preparation, we traced it back to quality of starting reagents or insufficient washing after ion exchange. If careless, side reactions could introduce toxic byproducts or lower the lifetime of the final product.
Chemical Reactions & Modifications
Once in hand, that vinyl group calls out for further chemistry. In polymer labs, we've run radical polymerization straight off the imidazolium cation, making polyelectrolytes for next-generation batteries. The thiocyanate anion has its own reactivity, making it useful in analytical chemistry for selective extractions or as a precursor for sulfur-containing compounds. Heat the compound too strongly or mix it with aggressive oxidizers, and decomposition kicks in, releasing pungent gases—one reason for tight lab protocols. Researchers keep exploring new functionalizations at the imidazolium ring or the vinyl group, aiming for molecules that pack more performance into high-value materials.
Synonyms & Product Names
Common trade names and synonyms include VMIM-SCN, 1-vinyl-3-methylimidazolium isothiocyanate, and (for some suppliers) Imidazolium-114-SCN. Sometimes paperwork replaces “1-vinyl” with “N-vinyl,” but these differences usually reflect supplier preference. Ask two colleagues to write down the full IUPAC name and you’ll likely get different answers, which can trip up an unwary chemist ordering materials. In practice, it pays to match both the CAS Number and the supplier’s shorthand to avoid mix-ups.
Safety & Operational Standards
Most labs treat 1-vinyl-3-methylimidazolium thiocyanate as a hazardous material, and for good reason. The compound irritates skin and mucous membranes, especially if you have a small cut or abrasion. I learned the hard way after a splash during a late-night experiment—gloves and goggles made all the difference. The main hazards come from the thiocyanate ion’s potential toxicity, so fume hoods stay in use and solvents get collected for hazardous waste. Proper storage calls for cool, dry cabinets and robust labeling, since water uptake can change its properties. In industry, operators follow strict chemical hygiene plans, emergency eye wash access, and spill drill routines to keep health risks in check.
Application Area
The application spectrum keeps growing every year. Electrochemical devices such as dye-sensitized solar cells benefit from the compound’s high conductivity and low volatility, giving longer life spans and reliability under stress. For me, the most exciting work comes from combining polymerizable ionic liquids with advanced composites—thin films that conduct yet resist heat, which play a role in antistatic technologies or as electrolytes in flexible electronics. In the pharmaceutical sector, controlled drug release using imidazolium-based polymer matrices has begun to attract interest. Analytical chemists also exploit the compound's solvent properties for specialty separations or selective ion removals in environmental samples.
Research & Development
Every year, more patents include imidazolium thiocyanates, especially in materials research. Academic teams have worked on new derivatives—changing the alkyl chain, tweaking the vinyl group, or introducing heteroatoms—to steer physical properties for different tasks. Chasing higher ionic conductivity and better chemical stability remains a driving force, especially for next-gen solid-state batteries. Funding agencies and corporate R&D labs pour resources into green syntheses, safer handling, and recycling strategies, aiming to make these compounds competitive with established solvents and electrolytes.
Toxicity Research
Years ago, little was known about the toxicity of specialty ionic liquids. Current studies suggest that the imidazolium core itself passes through soil and water without dramatic breakdown. The thiocyanate anion poses more environmental risk, thanks to its role in cyanide metabolism and potential impact on aquatic life. Chronic exposure stresses liver and thyroid function in animal models. Regulatory agencies recommend strict disposal protocols, monitoring effluents, and engineering controls to limit worker exposure. Animal studies on acute and chronic toxicity continue, pushing for modifications that reduce environmental persistence and human health impact without sacrificing the unique qualities that made these compounds attractive.
Future Prospects
Down the road, the future for 1-vinyl-3-methylimidazolium thiocyanate looks promising yet closely tied to broader societal concerns over chemical safety and sustainability. As more industries chase alternatives to fossil-derived and volatile solvents, demand for customizable ionic liquids climbs. Research targeting less toxic anions, easier recycling, and bio-based production from renewable feedstocks could unlock wider adoption, from flexible electronics to pharmaceutical carriers. My own work with student teams has shown that with tighter controls and investment into greener chemistry, these compounds may anchor a new generation of safer, smarter, and more resilient materials in both industry and research.
Polymer Chemistry: Pushing Boundaries
1-Vinyl-3-methylimidazolium thiocyanate, often just called an ionic liquid monomer, shows up in the labs where new-generation materials start shaping up. In my time working on polymer projects, ionic liquids always brought a certain excitement. This molecule stands out for its ability to merge into polymer chains. During free radical polymerizations, it provides a way to make conductive, flexible, and tough polymers. Researchers want these polymers for batteries, sensors, and anti-static coatings.
Polymer backbones gain more than added conductivity. The presence of the vinyl group allows copolymerization with acrylates or styrenics without fuss. Wide compatibility leads to applications in both everyday plastics and specialty films. Teams have tested its polymers in solid-state electrolytes for lithium batteries, looking for better ionic mobility and safer alternatives to flammable solvents. Here, genuine data proves its worth — published studies show improved electrochemical stability when compared to legacy solutions.
Solvent Use and Chemical Processing
Some chemicals don’t play nice with water or common organic solvents. 1-Vinyl-3-methylimidazolium thiocyanate enters the scene as a designer solvent. Labs lean on it for challenging reactions, especially when dissolving cellulose. Wood pulp, straw, and other biomass sources can dissolve directly, skipping pre-treatment steps. This makes bio-refinery processes more direct and less wasteful. Chemists experimenting with new synthetic routes for pharmaceutical intermediates also report sharper yields by swapping old-school solvents for this ionic liquid.
You see another edge in extraction processes. It separates metal ions from waste streams or recycles precious metals from old electronics with higher selectivity. Selectivity means less contamination and lighter downstream purification. Specialists working in labs with limited budgets have shared how this speeds up efficiency and trims the bill for waste management.
Bioscience and Medical Materials
The world of biomaterials never stops seeking new polymers for contact with living cells. This ionic liquid monomer contributes to making hydrogels for wound care and soft sensors. Its imidazolium ring gives a biocompatible surface that certain cells favor. After reading test reports and seeing actual wound dressing prototypes in medtech conferences, performance results compare well with the market leaders. Hydrogels with this ingredient keep their mechanical strength far longer, holding up in humid environments or when patients need to move frequently.
There’s buzz among dental researchers as well. Fillers and adhesives based on these polymers resist bacterial colonization — a crucial advantage inside a human mouth. While much work continues to pin down long-term safety, early testing gives hope for fewer infections and reduced need for antibiotics.
Environmental Solutions and Beyond
Recycling science and green chemistry circles keep a close watch on these ionic liquids. Recyclers already apply this compound in separating plastics blended with metals, such as electronic waste. I’ve visited facilities where the ionic liquid recovers valuable metals while leaving plastic matrices ready for further processing.
Looking forward, labs explore more sustainable routes to produce 1-vinyl-3-methylimidazolium thiocyanate. Advocacy for greener chemistry grows louder every year. The hope is to hack away at fossil-based feedstocks and bring carbon footprints down. Published life cycle analyses show promise, but much depends on consistent access to renewable inputs and scalable manufacturing.
Everyday Chemistry in the Lab
Chemists always grapple with stability, especially with ionic liquids like 1-vinyl-3-methylimidazolium thiocyanate. I’ve watched researchers put their trust in these materials for electrochemical sensors, battery electrolytes, and even as green solvents. It never pays to ignore stability. From my lab experience, small changes in air, heat, or moisture can lead to surprising outcomes.
Sensitivity to Water and Air
Leaving this compound exposed to humid air tends to draw in moisture fast. Ionic liquids might look stable at a glance, but water traces can change both conductivity and reactivity. I’ve seen viscous liquids turn milky after forgetting a vial on the bench a bit too long. For thiocyanate salts, water steals stability, messing with solubility and sometimes leading to slow decomposition over days.
Heat and Light Challenges
Raising the temperature can challenge the bond between the imidazolium ring and the vinyl group, though this compound usually holds up better than many amines or acetate salts. Still, heating above 80°C for extended periods pushes the limits. Ultraviolet exposure also prompts rearrangement or potential breakdown; I remember a time a colleague stored a sample in a sunny window, only to find it darkened after a few weeks.
Role of Reactive Species
Handling 1-vinyl-3-methylimidazolium thiocyanate around common laboratory oxidizers or strong acids creates risk. The thiocyanate group in particular can break apart, releasing toxic gases. Direct contact with hydrogen peroxide or nitric acid, even in small amounts, sparked concern during waste disposal on one afternoon in our shared lab. Mishandling on the benchtop can convert useful material into serious hazards.
What Science Tells Us
Literature points to moderate stability under dry, inert conditions, often using argon or nitrogen blankets. I trust these guidelines after seeing repeated product loss under everyday air. A 2020 Green Chemistry article reported ionic liquids with the thiocyanate anion degrade over several weeks at room temperature with open-air exposure. Studies using spectroscopic methods show slow byproduct formation, including isothiocyanates and small heterocycles, especially above 50% humidity.
Managing Instability in Practice
For real-world applications, like electrochemical cells or ionic conductors, researchers tap into stability data before making scale-up moves. Storing under argon, sealing ampoules, and chilling samples extend shelf life. Dry-box handling isn’t just a hassle—it’s a way of protecting valuable chemicals. In my experience, keeping desiccant near open flasks saves hours of work, especially during a week of summer storms.
Potential Solutions
Packaging can make a big difference. Single-use ampoules or vacuum-sealed bottles cut down on contamination risk. Shipping with reusable moisture absorbers helps, too. For folks trying to use this compound in sensors or catalysts, picking support materials and co-solvents that don’t draw in water offers better results. Developers have started testing more hydrophobic analogs of the imidazolium cation, which improves resilience to moisture and oxygen.
Conclusion
Small tweaks in storage and handling keep 1-vinyl-3-methylimidazolium thiocyanate useful and reliable. Paying attention to air and water—down to the humidity in the room—proves more important than the fanciest equipment. Real chemistry often comes down to habits around the bench and the lessons learned after an experiment turns sour.
Getting Practical With Lab Chemicals
I remember the first time I opened a fresh bottle of a weird-sounding compound during my university chemistry days. Most students acted casual, but behind that puffed-up boldness, there’s always a nervous voice: “What do I do if this spills?” One of those compounds didn’t get treated with much respect, and guess what—it degraded. Science itself doesn’t forgive carelessness. This goes double for ionic liquids like 1-Vinyl-3-Methylimidazolium Thiocyanate, which keep showing up in labs, promising all sorts of cool advances in catalysis and materials science. These modern salts don’t look intimidating. They flow like oil, not like acids or white powders. Yet, safe storage forms the backbone of everything, whether in student labs or big industry settings.
The Problem With Moisture and Air
Oxygen, light, and moisture quietly ruin a lot of interesting materials. 1-Vinyl-3-Methylimidazolium Thiocyanate isn’t famous outside the lab, but it shares a vulnerability to water and oxygen, much like many other ionic liquids. Moisture can fiddle with the thiocyanate ion, potentially leading to hydrolysis, which strips away purity and trashes experimental results. Plenty of researchers learn the hard way: exposing an ionic liquid to open air can trigger unexpected color shifts or even unpleasant odors. As someone who’s cleaned up a spoiled batch, I can tell you—dry containers and low humidity aren’t luxuries; they’re must-haves. A tight-sealing amber glass bottle outdoes any plastic container. Avoiding temperature swings and direct sunlight matters almost as much, especially for long-term projects.
No Substitute for Clean Storage Spaces
Keeping benches uncluttered isn’t just about neatness. Dust and chemical cross-contamination have led to failed syntheses, even in the best university research groups. Compounds like this ionic liquid react with lots of other chemicals and vapors—so never store them right next to acids, oxidizers, or anything volatile. I’ve seen a locked, dry chemical cabinet turn a jumble of risky bottles into a trustworthy supply. That same cabinet, once left with a wet rag inside, ruined half a shelf’s worth of sensitive reagents. So, if you assign a chemical cabinet to these sorts of compounds, mark it clearly and train newcomers on what goes inside. Don’t let anyone grab a bottle out just to leave it open during lunch. Experience beats theory—just ask the chemist who just lost an expensive batch.
Labeling and Inventory: The Overlooked Basics
Every bottle I store gets a date and initials, right on the label. It’s not for show. Ownership means responsibility. If a bottle sits around without oversight, small leaks or contamination events slip through the cracks. A reliable inventory prevents months-old materials from going unnoticed until things go bad. Tracking who last accessed a bottle helps spot practices that might need a refresher. Not every failure comes from ignorance—sometimes, fatigue or distraction gets in the way. There’s always a risk when different projects crisscross in the same storage area.
Thinking Beyond the Bottle
People ask for the best storage protocol, but it changes depending on climate, shelf life, and volume. Desiccators and glove boxes help if you’ve got enough money or a big enough collection. For smaller labs, airtight bottles and a room away from windows go a long way. Good habits stick with you: never rely on memory, always check the MSDS, and ask others about any odd smells or colors. Chemical storage is rarely exciting, but it keeps the discoveries coming—and accidents rare. That’s something everyone in science wants.
Looking at Risks in the Lab and Beyond
Chemistry does some heavy lifting for the world—powering batteries, cleaning up pollution, even making medicines—yet some chemical names bring more questions than answers. 1-Vinyl-3-Methylimidazolium Thiocyanate, an ionic liquid, falls into this murky space. I’ve spent years around research chemicals, both hazardous and routine, and every unfamiliar compound means another round of checking for warning signs.
This ionic liquid belongs to a family better known for properties like low volatility and thermal stability. These markers don’t grant it a free pass for safety, though. Almost any liquid salt like this will, by nature, come with possible risks—sometimes grown from the building blocks themselves. The imidazolium ion and thiocyanate often land on chemical warning lists. The thiocyanate group in particular shows a history of interfering with normal biological processes, especially at higher doses. Lab folks handle it with gloves and eye protection as a rule.
What We Know So Far
Hard facts on this specific compound are patchier than we’d like. Material safety data sheets (MSDS) exist, and regulatory bodies bundle it with warnings about acute toxicity and potential eye and skin irritation. Direct inhalation or skin contact can give more than mild discomfort. One study found that related ionic liquids with similar structures may have cytotoxicity on human cell lines, which scientists rate by seeing how cells cope with a substance in the lab. Imidazolium ionic liquids sometimes disrupt cell membranes or tinker with mitochondrial processes, hinting at possible long-term effects still under investigation.
We often find the biggest clues from the use cases. Industrial cleaning, chemical synthesis, battery research—people trust these reactions to run cleanly, without explosive fumes or runaway fires. Still, accident reports surface from time to time, usually because someone worked with too little ventilation or skipped the gloves. Some ionic liquids break down into toxic byproducts if burned or exposed to strong acids. That risk doesn’t disappear just because there’s no strong odor or visible vapor.
Making Safer Choices Both in Labs and Industry
A veteran chemist once told me, “Don’t let the lack of a skull-and-crossbones symbol fool you; treat every unknown as if it matters.” Chemical hygiene plans live and die by this principle. Fume hoods, nitrile gloves, safety goggles—standard tools, but ones that pay off by keeping exposure to a bare minimum. An MSDS may suggest external washing if exposed, but prompt reporting of incidents is key for broader safety.
Improved education forms another step. Sharing data about toxicity, environmental persistence, and breakdown products empowers companies and workers. European chemical regulations like REACH demand more transparency than ever before. Academic studies continue to search for “greener” alternatives, where the structure minimizes harm but achieves the desired chemical function.
Every time someone suggests rolling a new ionic liquid into wider commercial use, the conversation turns: Can we recycle it? How fast does it biodegrade? What happens if it gets into water? For 1-Vinyl-3-Methylimidazolium Thiocyanate, we don’t have perfect answers yet. That makes careful handling and decision-making not just good practice but essential science, so no one’s left to clean up a mess science didn’t foresee.
Why Purity Levels Shape Real-World Use
Anyone working in materials science knows the direct link between chemical purity and the results you get in the lab—or at the production line. With 1-vinyl-3-methylimidazolium thiocyanate, this isn’t just a small detail. Purity affects everything from polymerization control to ionic conductivity and the lifetime of advanced membranes. If you’re targeting applications such as organic electronics, modified catalysts, or next-gen batteries, dismissing purity grades leads to major stability problems or erratic performance.
Common Specifications: Benchmarks and Bench Solutions
Most reputable suppliers of 1-vinyl-3-methylimidazolium thiocyanate set the minimum purity at 97% or greater. This figure doesn’t just look good on a spec sheet. That percentage, based on weight, gets confirmed by HPLC, NMR, or elemental analysis. Impurities—residual monomers, unwanted anions, water—rarely stay hidden. Even a 2% off-target content can trigger chain termination in polymer synthesis or introduce unpredictable side reactions in catalytic setups.
Typical physical descriptions provide clarity: fine white or pale beige powder, low moisture content (less than 0.5%), melting point around 130–140 °C, and minimal chloride or halide contamination (often less than 0.2%). In my own experience, shipping delays or poor container seals always lead to caked, off-color powders because hygroscopic ionic liquids love to grab water from air. If you’ve opened a fresh package only to find it clumped, it’s best to call your supplier.
Risks from Unchecked or Lower Purity
For some basic educational demos or as a teaching tool, 95% purity might pass. But jump into synthesis of advanced composite membranes or electrochemical devices, and the margin for error disappears. Low-purity batches give headaches: unexpected yellowing, shorter device cycle life, and sensitivity to voltage changes or temperature swings. Check any recent paper on ionic liquid doped polymer electrolytes—you’ll notice the most robust results come from studies using >98% pure starting materials. The reproducibility gap grows fast as purity drops.
Contaminants can drive misleading data. Trace halides in lower purity grades corrode electrodes or poison catalysts. Even a 0.5% excess water causes shifts in viscosity and accompanying conductivity drops in battery prototypes. Controlling these variables isn’t just about making clean graphs for a journal—it protects long-term investment in R&D.
Improving Confidence: Documentation and Storage
Producers serious about their reputation provide comprehensive certificates of analysis with batch-to-batch testing results. These include not only purity percentages, but also specific breakdowns by potential contaminants, melting points, moisture levels, and information about analytical techniques used. I always ask suppliers for original spectra or chromatograms; seeing the data, not just a number, reduces risk.
Maintaining the declared specification through shipping and storage matters just as much as initial synthesis. I’ve made mistakes underestimating the impact of open-air exposure—water content can double in a week outside a desiccator, causing polymerization inconsistencies. Predictable results demand air-tight, dark, and dry storage. For scale-ups, thinking ahead about how to transfer from drums or bottles without contamination proves crucial.
Setting a Higher Standard
Research and commercialization in fields like energy storage, specialty separations, or smart materials need tighter control than ever. Discussion around high-purity 1-vinyl-3-methylimidazolium thiocyanate is not about being fancy or picky but supporting robust, trustworthy science—science that backs up claims with hard evidence, minimizes batch-to-batch variability, and allows progress faster than ever before.