10-Aminodecanoic Acid: Insights, Uses, and Future Potential
Historical Development
10-Aminodecanoic acid stepped onto the stage of synthetic chemistry in the mid-20th century, sparked by the demand for specialized building blocks needed in polyamide and nylon synthesis. Chemists recognized that the ten-carbon chain and terminal amino group create a versatile foundation for experimentation. Researchers expanded its applications over the decades, linking its structure to a range of fibers, biomedical materials, and surface coatings. The journey from laboratory curiosity to industrial staple reflects persistence from both industry and academia in chasing new materials. Early research focused on unlocking efficient synthetic routes, as original methods stalled at low yields and high costs until catalytic hydrogenation and more selective protective group strategies emerged.
Product Overview
10-Aminodecanoic acid doesn’t gather mainstream attention, yet those who work in polymer science or the chemical industry depend on it for specialty applications. It resembles a colorless to off-white powder with a mildly amine-like odor, supplied in technical and high-purity grades. Suppliers cater to different customer backgrounds, shipping this chemical to laboratories seeking advanced building blocks for new polyamides, anti-corrosive coatings, or as a starting material for custom surfactants. The availability of both laboratory and industrial quantities ensures researchers and manufacturers can test new concepts without facing uncertain supply lines.
Physical & Chemical Properties
10-Aminodecanoic acid looks like a standard crystalline solid on the surface, but its combination of a terminal amine and carboxyl group gives it unique solubility and reactivity. Its melting point generally sits around 165–169°C. The density stands close to 1.0 g/cm³. The long alkyl chain means limited solubility in water but increased compatibility with organic solvents. The molecule’s amphiphilic character lets it anchor itself in both hydrophobic and hydrophilic matrices, making it a useful intermediate for specialty surfactants and copolymers. Both the terminal amino and carboxyl functional groups allow easy downstream modifications.
Technical Specifications & Labeling
Producers often list this compound at purity levels above 98%, with trace metal and residual solvent limits for higher-end applications. The acid value runs parallel to the carboxyl functionality, while total amine content is checked to confirm molecular integrity. On technical data sheets, batch number, manufacturing date, and storage recommendations appear as standard; this ensures traceability and reduces the chance of degradation from humidity or excess heat. Regulations require safety warnings about skin and respiratory sensitization, so labels mention proper PPE requirements and first aid recommendations.
Preparation Method
Industrial synthesis typically begins with decanoic acid or its esters. Through nitrile intermediates, hydrogenation brings the cyano group to an amine, followed by hydrolysis when necessary. Improvements in catalyst technology have given chemists more precise control over chain selectivity, reducing byproduct formation and streamlining the purification process. Lab-scale methods lean on protection and deprotection chemistry, using Boc or Fmoc groups to shield the amine before carboxyl group derivatization. Each step demands close quality monitoring, since even trace impurities can interfere with downstream polymerization or bioconjugation.
Chemical Reactions & Modifications
Chemists regularly convert 10-aminodecanoic acid into amides, esters, and urethanes, opening pathways toward diverse specialty plastics and functional coatings. The amine group reacts with acid chlorides, isocyanates, and anhydrides, allowing tailored functionalization. Oxidation, reduction, and alkylation extend the range even further. One researcher’s experience in pharmaceutical applications involved anchoring this molecule to solid supports for peptide synthesis. Its properties handle repeated washing and pH changes in these workflows, standing up to both acid and base treatments. In polymer blends, its reactivity boosts compatibility between hydrophilic and hydrophobic phases.
Synonyms & Product Names
Depending on the source, this compound might turn up under several names: 10-aminocapric acid, 10-amino-n-decanoic acid, or decanoic acid, 10-amino. Custom suppliers may designate proprietary catalog numbers or blend names if it shows up in prepackaged mixtures for specialty coatings. Researchers sometimes truncate it to ADAA, though consistent naming helps prevent confusion, especially when handling related omega-amino acids with different chain lengths.
Safety & Operational Standards
Working safely with 10-aminodecanoic acid means treating it with the same respect as other organic amines. Its dust can provoke mild skin and respiratory irritation; gloves, eye protection, and local exhaust ventilation are standard safeguards in labs and factories. Some process technicians mention mild headaches or rashes from direct exposure without proper PPE. Spill cleanup uses absorbent pads and inert materials, while storage relies on tightly sealed containers away from strong acids and oxidizers. Regulatory agencies in North America and Europe recommend hazard communication training for workers who regularly weigh, mix, or transfer the material. Good records and safety data sheets allow employers to trace exposures and implement medical surveillance if health questions arise.
Application Area
Manufacturers of high-performance polyamides depend on 10-aminodecanoic acid for making tough fibers and plastics. These polyamides show up in automotive parts, machine tools, conveyor belts, and medical device housings, where strength, flexibility, and resistance to heat or chemicals matter. Biomedical engineers leverage its functional groups for drug delivery scaffolds, hydrogels, or even tissue engineering templates. Surfactant developers see potential for materials with better oil–water interface control and reduced environmental persistence. In coatings, chemists add it to improve anti-corrosive properties or promote adhesion between dissimilar materials.
Research & Development
R&D teams rarely stand still. Over the years, I’ve seen polymer labs trial new copolymers that incorporate 10-aminodecanoic acid for improved elasticity and solvent resistance, especially for next-generation automotive or aerospace composites. In medical technology, teams test it for biodegradable implants that break down predictably within the body. Universities pursue ways to graft this molecule onto nanoparticles, aiming for precision drug delivery or advanced biosensing. Young chemists value it as a model amino acid for testing new peptide-based reactions, since the ten-carbon linker mimics many naturally occurring fatty chains.
Toxicity Research
Animal models and cellular studies show that acute exposure to 10-aminodecanoic acid produces low toxicity, though high doses can irritate skin, eyes, and perhaps the gastrointestinal tract. Chronic exposure studies remain limited, as the material mainly sees use in industrial rather than consumer settings. My review of European Chemicals Agency filings turned up no record of carcinogenicity, mutagenicity, or reproductive risk at workplace exposure limits. Still, companies err on the side of caution with mandatory barrier protections, emergency eyewash stations, and air monitoring. Researchers call for more studies on environmental persistence and long-term ecological effects, given the growing use in water-soluble or dispersible polymer systems.
Future Prospects
The outlook for 10-aminodecanoic acid links industry need for tough, functional materials with environmental stewardship. New bio-based synthesis methods crop up, targeting feedstocks from renewable oils instead of petroleum, which should shrink the carbon footprint and appeal to green chemistry advocates. Advances in surface modification could make polyamide blends more recyclable or biodegradable, granting designers both performance and end-of-life sustainability. In healthcare, innovation teams chase smarter drug carriers and tissue supports that rely on the acid’s unique balance of flexibility, strength, and chemical reactivity. Upstream, automation and continuous-flow manufacturing promise faster and cleaner synthesis, shrinking both cost and environmental burden. Across sectors, partnerships between companies, universities, and regulators promise a future where 10-aminodecanoic acid underpins a new round of safer, stronger, and more sustainable material technologies.
From Chemistry Lab to Living Room
10-Aminodecanoic acid looks technical on paper, but its impact stretches far beyond laboratories. People often run into it without even knowing—touch a nylon carpet or zip up a jacket, and you’ve brushed against this chemical’s handiwork. This molecule, once isolated and purified, helps shape nylon-11, a type of plastic that’s strong, flexible, and resistant to chemicals. Scientists discovered its key role decades ago, and since then, its influence in our daily lives only grows.
Improving Everyday Products
Ask anyone in the industry why nylon-11 matters, and you’ll likely hear stories about durability, flexibility, and the ongoing battle to build better products. From automotive fuel lines to flexible electronic casings, 10-aminodecanoic acid keeps popping up as the star ingredient. That matters because nylon-11 doesn’t just offer resilience—it also handles temperature swings well and shrugs off harsh chemicals. Imagine a car part that won’t crack on a winter morning or a smartphone cable that resists oils, salt, and sunlight for years. That reliability makes life easier for engineers and everyone else down the line.
Environmental Choices on the Table
We face mounting challenges with plastic waste and fossil fuels. The appeal of 10-aminodecanoic acid lies not only in its toughness but also in where it comes from. Big industrial players usually make it from castor oil, a renewable plant source, instead of petroleum. This shift lowers a company’s carbon footprint—every little bit counts these days. For folks with a strong interest in sustainability, that connection to crops rather than drilling has real value.
Addressing Barriers and Looking Forward
Raw materials, even from renewable sources, can spike in price or run short. Supply hiccups lead to cost headaches, and small shifts can ripple across whole industries. If factories rely too much on one crop, diseases or drought can halt production. People in the field know to keep an eye on global agricultural trends and invest in research for alternative feedstocks. Spreading risk across different sources—perhaps from algae or waste byproducts—guards against sudden shortages.
Building Knowledge and Trust
People still ask, “Is this chemical safe?” That’s an important question, especially as awareness grows about health and sustainability. Research shows that 10-aminodecanoic acid and its products don’t leach toxins into the environment or threaten workers when handled correctly. Regulators and manufacturers bear responsibility for upholding high safety standards. Enthusiasts and watchdog groups often call for more open reporting, which builds public trust and gives families peace of mind.
Toward Smarter Plastics
The future of 10-aminodecanoic acid hinges on a mix of smart policy, public awareness, and unrelenting curiosity. Chemists keep looking for easier, cleaner ways to make it. Companies explore blending it with biodegradable materials. Teachers and journalists share real stories about breakthroughs and setbacks, opening the topic to more voices outside the ivory tower. Whether you’re concerned with planet health or the electronics on your desk, this molecule and its journey have a place at the table.
What is 10-Aminodecanoic Acid?
10-Aminodecanoic acid, sometimes called 1-aminocapric acid, turns up in the world of specialty chemicals. Chemists know it as a building block for nylon-10 and other specialty polymers. Curious minds have asked if it belongs on ingredient lists for drugs or skin creams. A quick look reveals 10-aminodecanoic acid as a medium-chain amino acid with a primary amine at one end and a carboxylic group at the other.
How Science Gauges Safety
Pharmaceuticals and cosmetics answer to some of the toughest safety rules. Both the European Medicines Agency and the U.S. Food and Drug Administration ask companies to show that ingredients don’t harm people—even before anything reaches a shelf. Toxicology studies, cell tests, animal models, and chemical analyses shape the picture. Regulators want to know if a molecule irritates skin, causes allergic reactions, builds up in tissues, or mutates DNA. Sometimes chemical structure gives clues, but that’s just a starting line.
Current Evidence on 10-Aminodecanoic Acid
Published research on 10-aminodecanoic acid’s use with humans stays limited. Unlike dozens of common cosmetic ingredients, this acid rarely pops up in ingredient catalogs from big players in pharma or beauty. Right now, this stands out. Usually, when a substance is missing from safety databases, it means chemists haven’t tested it on skin or with live cells beyond basic research. Studies looking at polymers built with 10-aminodecanoic acid focus on physical properties for plastics, not what happens when applied or ingested by people.
Few signs point to major irritant effects in small-scale studies, and amino acids in this chain-length range tend to break down in typical ways in the human body. Still, nobody has published thorough, peer-reviewed studies on contact allergies, chronic exposure, or long-term metabolism of pure 10-aminodecanoic acid in humans. The European Chemicals Agency lists it as a substance requiring more information for proper hazard classification.
Why This Matters
Consumers today have become far more careful about what touches their skin or goes in their bodies. I’ve spoken with parents, patients, and people with sensitive skin who now Google every ingredient before buying. In my time working in community health, I’ve seen allergic reactions and rashes arise from unlikely culprits. For any novel raw material, people want clear evidence instead of guesses. Social media spreads fear fast if a new compound isn’t well-understood.
Transparency about ingredient safety builds trust—not just with the public but with regulators and physicians. Uncertainty around a rarely studied ingredient weakens public confidence and opens the door for misinformation. Product recalls from overlooked irritants or contaminants quickly erode consumer faith.
Moving Toward Assurance
Chemical companies and manufacturers interested in 10-aminodecanoic acid should fund proper studies. Basic tests need to cover skin irritation, phototoxicity, mutagenicity, and breakdown in the body. Publishing findings, not just keeping results in internal files, would help reassure everyone from doctors to ingredient-conscious shoppers. Any step toward clinical use or topical application should include robust, published data reviewed by independent toxicologists or dermatologists.
Open data, active sharing of negative as well as positive results, and clear language about both risks and uses serve everyone better. If future studies confirm that 10-aminodecanoic acid carries no hidden dangers, maybe it earns a spot alongside other amino acids in safe formulations. For now, skepticism stays justified. People deserve more data before this molecule gets a routine place in healthcare or beauty products.
Chemical Structure That Counts
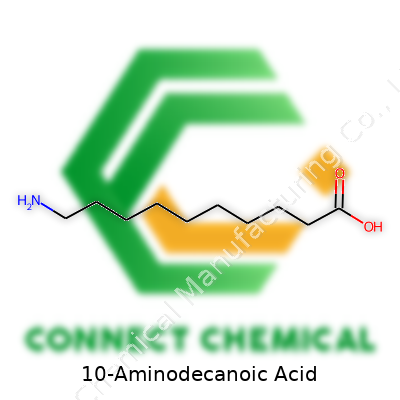
Here’s a compound with a name that might not roll off the tongue but shows up in some fascinating places, especially in polymer chemistry. 10-Aminodecanoic acid features a backbone of ten carbons in a straight line. One end sports a carboxylic acid group (–COOH); the other end holds a single amine (–NH2) group. If you drew it as a formula, it’d be NH2–(CH2)9–COOH. This combination of groups at either end turns the molecule into a building block with a lot of flexibility. The amino part grabs attention from those making nylon-based materials, because it offers a way for long chains to snap together efficiently.
Molecular Weight and What It Tells Us
You can’t ignore numbers in chemistry. 10-Aminodecanoic acid clocks in at a molecular weight of 187.29 g/mol. That’s neither very light nor especially heavy for a simple organic molecule, but it puts it in range to act as a handy monomer in bio-based plastics and specialty polymers. This sort of information isn’t just trivia for a chemist; it matters for production planning, shipping requirements, and energy needs in manufacturing. Every gram adds up when scales tip from lab experiments into industrial vats.
Why Structure and Weight Matter
Polymers, plastics, and functional materials all draw on simple molecules like this one. I’ve watched companies turn a powdery pile of 10-aminodecanoic acid into durable nylon fibers or smooth coatings. The structure—long enough to provide backbone flexibility, with those reactive ends—explains a lot about why it’s been picked for bio-based materials. Years ago, a colleague pointed out that the straight ten-carbon chain gives an ideal mix of strength and processability; shorter chains can make things brittle, while longer ones push up melting points until you need hotter equipment that burns through energy budgets.
The amine group, sitting opposite the acid, opens up condensation pathways. You can link amines to acids head-to-tail in polymerization, giving predictable chain lengths and, eventually, reliable finished parts. Nylon-10, for instance, uses this molecule directly. Growing up around folks who worked in textiles, I saw how the move from petroleum-based to more renewable monomers got a boost from this kind of chemistry. Each part of the molecule serves a purpose, making it more than just a collection of atoms.
Looking Toward Better Outcomes
With sustainability taking a bigger role in industry decisions, 10-aminodecanoic acid stands out for another reason. Chemists have tweaked processes to produce it from vegetable oils—a renewable resource—rather than rely on fossil feedstocks. This switch can shrink carbon footprints and align with regulatory pressures. Experience shows that companies able to pivot to bio-based raw materials often find new partnerships and grow market share, especially as consumers and clients flag those green credentials.
Of course, cost and scalability keep everyone on their toes. 10-Aminodecanoic acid isn’t as cheap as older petrochemical monomers, but advancements in biotechnology and fermentation may narrow that gap. Research groups from Europe to Asia are racing to scale up production without driving up costs. The chemical’s well-mapped structure, known molecular weight, and proven utility in making safer, cleaner plastics keep it relevant as both science and manufacturing shift toward smarter, cleaner workflows.
Why Storage Matters with 10-Aminodecanoic Acid
Working with any chemical always calls for practical attention, not only for safety but also to protect the pure form of what’s inside each container. 10-Aminodecanoic acid, a specialty compound often found in the plastics and coatings industries, only does its job when it stays stable on the shelf. An unstable container quickly becomes dangerous, so those of us with experience in labs and warehouses have learned to respect every aspect of storage.
Storing the Chemical: Real-World Practices
Placing 10-aminodecanoic acid on a shelf in an open workspace defeats the purpose of every safety training session I’ve ever attended. This material holds up best in a tightly sealed container, preferably made of high-density polyethylene or another compatible polymer. Glass can also work, but only if storage accidents never happen—something I won’t bet on.
Setting the container aside in a cool, dry, well-ventilated place helps avoid clumping or unwanted reactions. Humidity never does chemicals any favors; water can trigger hydrolysis or messy clumps, which undoes product quality and may generate unpleasant fumes. Personal experience tells me that old shelves near radiators or sunlight often ruin perfectly good stock. A consistently temperate storeroom extends shelf life and saves money, especially with a compound rarely bought in bulk. Fluctuating heat and cold can break down its molecular stability. The risk isn’t just waste—it’s potential spills and health hazards for anyone nearby.
Handling Precautions: No Shortcut Here
For anyone handling 10-aminodecanoic acid, gloves and eye protection aren't suggestions—they’re vital. The powder and dust easily irritate skin and eyes. I’ve watched colleagues skip goggles, then end up with a watering eye and an unexpected trip to the sink. Disposable nitrile gloves, goggles, and a lab coat form my basic setup on every shift. For larger amounts, a chemical-resistant apron and face shield raise the safety standard.
Avoiding dust clouds matters just as much in a shared workspace, so pouring and weighing should always happen under a fume hood. Even a minor spill spreads fine particles, leading to skin contact or accidental breathing in. Based on what I’ve seen, ignoring ventilation is like inviting trouble. Anyone who’s worked in cramped quarters with little airflow knows the headaches or sneezing that follow chemical exposure.
Spill Management and Waste
Accidents do happen. Small spills clean up fast with dry absorbent material—no water. Wiping up with a wet cloth might seem fine at first, but moisture creates slippery spots and can change the chemical’s structure. Bag the waste up as hazardous and label it properly. Hazardous waste regulations exist for a reason, and fines for improper dumping add up, not to mention the danger for maintenance staff.
Potential Solutions for Better Safety
Regular in-person safety training covers far more ground than online modules. I’ve noticed that walking through spill drills or hands-on container checks reduces mistakes by a lot. Every facility storing 10-aminodecanoic acid should have a clear policy—old containers clearly labeled, material safety sheets up to date, regular audits to spot leaks and worn packaging before issues start. Improving ventilation and automating some dispensing steps also limit direct contact, giving everyone peace of mind while working.
The bottom line is that 10-aminodecanoic acid won’t cause problems if you respect its chemistry and remember the basics: sealed containers, stable storage, protective gear, and fast action on spills. After years spent working with specialty chemicals, I see these rules less as burdens and more as guarantees everyone goes home healthy at the end of the day.
Nylon 11 and Plastics Manufacturing
Few people outside of the chemical industry recognize 10-aminodecanoic acid, but this compound has shaped how we live and work. The most prominent example is Nylon 11, a strong and flexible plastic built for performance. Industries rely on Nylon 11 to create tubing, cable coatings, and parts for electronics—things that face rough handling or exposure to grease. I’ve seen these materials in automotive shops, factory floors, and even the garden shed. Their toughness might not spark conversations at the dinner table, but you notice the difference when cables don’t crack in the cold.
Nylon 11 stands out for its resistance to fuel and chemicals. In the car industry, fuel lines face constant attack from oil, gasoline, and temperature swings. Using 10-aminodecanoic acid to make Nylon 11 solves these headaches, cutting down on leaks and repairs. I’ve talked with mechanics who remember cheap tubing that failed too soon. Now, the upgrade to Nylon 11 is a relief for anyone tired of spill cleanups.
Biodegradable Polymers and Medical Devices
10-Aminodecanoic acid pops up in fields that care about both strength and safety. Makers of medical tools and implants look for plastics that the body can handle. Research labs work on biodegradable versions of nylon derived from this acid. These innovations matter when producing sutures that gradually break down or drug delivery capsules that release medicine as they dissolve. Hospitals benefit when tools both function well and reduce waste. This blend of chemistry and healthcare carries the promise of safer recoveries and less worry about lingering materials inside the human body.
Coatings and Adhesives in Electronics
Phones, sensors, and connected gadgets depend on tiny wires and circuits that need protection from moisture, dust, and heat. Nylon 11 gets the job done here, too, thanks to the building blocks supplied by 10-aminodecanoic acid. Instead of brittle covers or coatings that peel away, these specialized plastics guard sensitive components for years. Engineers at tech companies pay attention to the raw materials, since longer gadget life cuts down on electronic waste. Anyone frustrated by frayed charging cables should see the value in materials tough enough to handle daily use.
Eco-Friendly Innovations
Sustainability now gets just as much attention as strength. 10-Aminodecanoic acid offers hope in the push for greener plastics. Some producers extract it from castor oil, not fossil fuels. Switching to plant-based resources shrinks a company’s environmental footprint. Farmers can find new markets for their crops while manufacturers meet tighter environmental standards. More companies want traceable supply chains that favor renewable sources. This shift lines up with customer demand for products that do less harm to the earth.
Challenges and Looking Forward
Access to cost-effective, high-purity 10-aminodecanoic acid means businesses can experiment with new blends and greener options. Investment in research fuels more breakthroughs, especially around recycling and plant-based processing. Groups advocating for circular economies push manufacturers to develop biosourced or even biodegradable plastics. The drive for better materials lines up with new policy and market expectations.
I’ve watched as small changes in material sourcing led to big ripples—stronger parts, healthier people, cleaner communities. The story of 10-aminodecanoic acid keeps unfolding, shaped by questions about what we need from our products and what planet-friendly innovation demands.