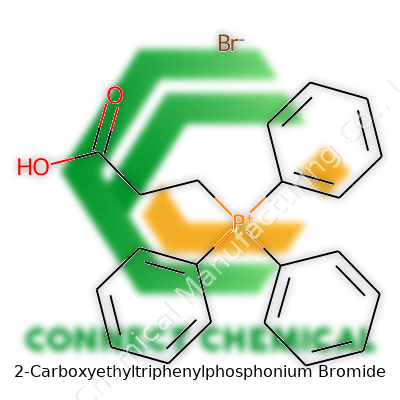
2-Carboxyethyltriphenylphosphonium Bromide: Depth, Detail, and Direction
Historical Development
Chemistry keeps finding ways to create new building blocks, and 2-carboxyethyltriphenylphosphonium bromide proves that the industry learns from the old while exploring the new. Organophosphorus compounds picked up steam in the 20th century once synthetic chemistry started branching out from simple reagents to specialized molecular tools. A handful of historic papers traced the groundwork for quaternary phosphonium salts, especially with the work of Wittig and his peers, whose discoveries back in the mid-1900s made the ylide reactions famous. Out of this foundation, lab groups discovered that adding a 2-carboxyethyl chain to the classic triphenylphosphonium backbone gave chemists a versatile and reactive salt, perfect for exploring modifications on carbon frameworks. Whatever breakthroughs happen today started with the same curiosity that drove those early pioneers in Europe and North America, and each new generation learns something from their careful stepwise refinements.
Product Overview
If you’ve handled specialty reagents in an organic chemistry lab, you know what to expect from 2-carboxyethyltriphenylphosphonium bromide. This is a white to off-white solid, offering both crystalline stability and a handy source of reactive phosphonium species. Sourced routinely from chemical suppliers, the salt arrives dry, often sealed for moisture protection. Its pricing and sourcing reflect ongoing demand in synthetic chemistry, particularly for applications where carbon–carbon bond formation takes priority over commodity chemicals. I remember opening a bottle for a graduate synthesis project, the faint but unmistakable chemical scent reminding me to keep a careful approach. This compound doesn’t sit in every undergraduate storeroom, but research and pharmaceutical labs consider it an essential piece, especially as peptide and advanced small molecule synthesis continue to grow.
Physical & Chemical Properties
Looking up at the periodic table doesn’t help much when you handle this salt—it sits well outside the usual sodium chloride routine. Its formula, C21H20BrO2P, spells out an interesting structural balance: three phenyl groups crowding a phosphorus center, set off by a bromide anion and a 2-carboxyethyl group sticking out. Melting points hover around 240–245°C, signaling both purity and robust crystalline bonds. It dissolves in many polar organic solvents but resists breakdown in pure water, a feature that speeds up reactions with less solvent fiddling. On the shelf, it prefers dry environments, since the salt can draw moisture and lose its free-flowing texture. Storage under an inert atmosphere keeps it at peak reactivity, an important lesson every time a bottle stays open too long and clumps form—something I’ve seen turn a promising sample into a frustrating mess.
Technical Specifications & Labeling
Industry standards set tight tolerances for this bromide: reputable bottles carry purity certificates with values above 97%, often determined by HPLC or NMR. Labels from trusted suppliers always underline hazard classes, batch numbers, preparation date, and storage recommendations—details that matter if people need traceability or accident reporting. Many suppliers reinforce their reputation with comprehensive data sheets, packing dense regulatory guidance into the delivery. If QC teams spot discoloration or an off-odor, procedures call for an immediate check, since even minor contaminants can derail reactions with such selective reagents. Every chemist working with regulated or documented synthesis routines should get a full rundown on these specifications before opening the cap.
Preparation Method
Setting up 2-carboxyethyltriphenylphosphonium bromide in the lab involves a classic approach: quaternization of triphenylphosphine with a suitable bromoacid, usually 3-bromopropionic acid. You mix and stir under mild reflux, giving the phosphorus center a direct, one-step path to the final quaternary salt. Purification usually goes by crystallization from ethanol or another alcohol, depending on the batch scale and the purity needed. Those who scale up beyond a few grams quickly learn that yields and purity hinge on patience, reaction monitoring, and clean glassware. Skipping a careful recrystallization often lands labs with sticky, hard-to-handle byproducts. Each synthesis run reminds workers why protocol documentation saves time, since otherwise, simple missteps threaten to throw off both reproducibility and costs.
Chemical Reactions & Modifications
Chemists love this phosphonium salt for its predictable, but versatile, reactivity. The classic Wittig reaction comes to life when you mix the salt with a strong base, usually sodium hydride or potassium tert-butoxide. This quickly forms the reactive ylide, which then jumps at aldehydes or ketones, creating new double bonds—crucial steps in many drug and natural product syntheses. That carboxyethyl tail doesn’t just sit there either; under the right conditions, you can transform it into esters, amides, or even protect it for downstream steps. Working with this bromide has taught me to keep a close eye on both pH and solvent, since even small changes in reaction setup can shift yields or selectivity, sometimes sending hard-won intermediates off to side products. Still, the salt reprents a reliable platform for method development and scale-up, and its broad utility makes it a staple for reaction optimization studies.
Synonyms & Product Names
In catalogs and lab books, the compound pops up under several alternate names. You might see listings as β-Carboxyethyltriphenylphosphonium Bromide, Triphenyl(2-carboxyethyl)phosphonium Bromide, or even more technical designations like (2-Carboxyethyl)triphenylphosphanium Bromide. Regulatory agencies log the compound under CAS number 4771-41-3. Getting familiar with these synonyms spares confusion during ordering or literature surveys, since even seasoned chemists occasionally stumble over the varied naming customs used across journals and suppliers.
Safety & Operational Standards
Real lab safety lessons often start with overlooked mistakes, and 2-carboxyethyltriphenylphosphonium bromide offers no exception. Gloves, eye protection, and a working fume hood matter every time, since organophosphorus compounds can pose toxicity concerns if handled carelessly. MSDS sheets always warn of skin, eye, and respiratory irritation; direct ingestion or exposure brings out stronger warnings. Spills call for swift, contained cleanup, but waste goes straight to hazardous bins—down-the-drain protocols would risk both lab safety and local compliance. Emergency protocols focus on thorough decontamination, quick reporting to lab supervisors, and medical consultation if necessary. I’ve seen students pay for a single forgotten glove by hours of paperwork and minor burns, so there’s no trade-off between speed and safety when dealing with this salt.
Application Area
Most of the real action happens in research labs and commercial synthesis routines focused on fine chemicals. Medicinal chemists use the bromide in the creation of unsaturated fatty acids, drug intermediates, and various natural products. Its role as a stable source of Wittig ylides lets scientists assemble carbon frameworks with precision—the backbone of many pharmaceutical ingredients and advanced materials. Teams working in polymer science and agrochemicals also experiment with the salt for quick access to functionalized monomers. In one project, I saw a collaborative group employ this phosphonium salt to model greener, one-pot syntheses, cutting down solvent use and waste loads. The compound’s flexibility and clean, targeted reactivity make it a favorite for those aiming to streamline multi-step organic syntheses.
Research & Development
Researchers keep finding new uses for 2-carboxyethyltriphenylphosphonium bromide. Synthesis techniques have pushed reaction times down, cut waste, and tuned yields higher than ever. Teams covering catalysis now eye phosphonium salts for transforming simple aldehydes into valuable α,β-unsaturated acids or esters. Data from major pharmaceutical labs suggest that optimizing Wittig parameters with this reagent leads to dramatic improvements in selectivity, reducing the cost and labor involved in isolating the right stereoisomers. Some groups dive into computational chemistry, mapping the electronic structure of ylides formed with the salt and linking this to observed reactivity trends. Academics and industrial chemists both know the value of robust, reproducible reagents, and this compound earns its bench space in a crowded chemical universe.
Toxicity Research
Toxicity checks reveal an important story with every new compound. Data for 2-carboxyethyltriphenylphosphonium bromide show moderate oral toxicity in animal studies—enough to demand healthy respect, not recklessness. Chronic exposure risks include organ impacts and possible carcinogenicity, but as with most specialized reagents, real harm emerges from repeated mishandling or accidents, not single, careful uses. Regulatory bodies in the US, Europe, and Asia keep updated safety data sheets, requiring proper labeling and special disposal protocols. Each new batch released for commercial or academic research receives fresh scrutiny and documentation, keeping both users and downstream communities safer.
Future Prospects
The demand for fine-tuned synthetic reagents never backs down, and 2-carboxyethyltriphenylphosphonium bromide stands to play a bigger role going forward. As synthetic methods lean toward automation, greener processes, and sharper selectivity, the practical lessons learned from decades of use offer real guidance. Research into sustainable solvents, better recycling of phosphonium wastes, and the integration of advanced analytics will drive the next chapters. Efforts around process intensification can simplify multistep preparations, shortening campaigns for active pharmaceutical ingredients and complex organic molecules. If the research community steps up investment in scalable, low-impact synthesis using such building blocks, the compound could see broader use in everything from bioactive compounds to innovative materials, offering practical paths to new science and better products for the next generation.
Why the Structure and Formula Matter
Chemists love learning about the nuts and bolts of a molecule before they ever weigh the powder. 2-Carboxyethyltriphenylphosphonium bromide packs quite a mouthful into its name, but it also packs a punch in the lab. Anyone who works with Wittig reactions knows this compound by heart. The formula—C21H20BrO2P—isn't just a random string of letters and numbers. It captures the entire essence of the molecule, from the number of aromatic rings right down to the heavy presence of bromide stuck to the structure.
The Details in the Formula
Every part of that formula plays a role. All those carbons? They're not scattered. Triphenylphosphonium brings three benzene rings attached to a central phosphorus, which gives it a bulky and very recognizable backbone. The phosphorus atom isn’t just decoration—this is the reactive heart, the site where chemistry usually happens. That little ‘carboxyethyl’ side chain—connecting through a simple ethylene bridge—brings in an acidic group, swinging chemistry possibilities wide open. Bromide as a counterion balances out the charge, making the whole thing a stable salt for storage and handling.
Why It’s More Than Just Numbers
Knowing the formula isn’t about reciting facts. In real life, a chemist needs the formula for calculations, predictions, and safety. Calculate molecular weight wrong, and your synthesis either falls flat or causes headaches. C21H20BrO2P carries a molecular weight over 415 g/mol, a detail you can’t overlook if purity or accurate dosing matters. Lacking this information, students can mess up yields, professionals might see runaway costs, or accidents waiting to happen. Those details build trust in research—no room for sloppiness, especially when people depend on reliable data for pharmaceutical or industrial work.
Applications and Considerations
Walk into any organic chemistry lab, and 2-Carboxyethyltriphenylphosphonium bromide usually waits in the fridge for the next round of Wittig reactions. The formula guides chemists through complex transformations, like converting aldehydes or ketones into alkenes with specific side chains. A missing oxygen or a stray hydrogen and all the planning goes sideways, wasting time and resources. The precision of the formula stands as a safeguard against confusion. During scale-ups, environmental health and safety folks also pore over it to flag hazards—bromide ions, aromatic groups, or anything else that could present risks if mishandled.
Steps Toward Safer and Smarter Work
Chemical formulas don’t fix process safety all by themselves. Teams use the formula to create safety sheets, waste management protocols, and spill plans. If a spill happens, the company already knows what they’re dealing with—part organic, part inorganic, and something that calls for gloves and maybe a fume hood. Responsible handling keeps everyone at ease. And in workplaces that train students or young chemists, teaching the exact formula means training the next wave of responsible scientists. Memorizing C21H20BrO2P goes far beyond passing a test—it helps build habits that protect health, conserve resources, and keep the science moving forward.
Navigating the Chemistry Lab with Caution and Curiosity
Every time I set foot in a chemistry lab, I see shelves stacked with bottles marked with tongue-twisting names. Among them: 2-Carboxyethyltriphenylphosphonium Bromide, a name that baffles some and excites those who love the gritty reality of chemical synthesis. As an organic chemist, I’ve reached for this compound more than once, not for its complexity, but for the simple solutions it helps construct where other options fall short.
But What Does It Do?
This compound often steps into the spotlight in the world of organic synthesis. It serves a specific role in Wittig reactions, a backbone method for building carbon-carbon double bonds in pharmaceuticals, agrochemicals, and materials science. Making an alkene in a targeted way is no small feat, and 2-Carboxyethyltriphenylphosphonium Bromide carves a unique path: its phosphonium salt helps deliver those double bonds with precision.
The magic trick here comes from its carboxyethyl group. This tweak lets chemists build molecules that would be frustrating or even impossible with plainer phosphonium salts. Take it from someone who has tried the alternatives—using this specific salt lets a scientist combine functional groups that usually wouldn’t play nice together. That’s opened doors for the design of anti-cancer agents and heart medications, among other things.
Bringing Flexibility to Drug Discovery
Doctors and patients count on chemists to push the boundaries of what a molecule can do. One application I’ve seen in the lab: this phosphonium bromide streamlines routes to complex drug scaffolds. In the late stages of a synthesis, dropping in a group that handles extra stress—like a carboxylic acid—upgrades the process. Instead of laboring through extra protection and manipulation steps, a straightforward Wittig reaction gets the job done. That means medicines hit the clinical stage faster, with fewer side reactions and less chemical waste. Speed matters for everything from pandemic response to novel cancer therapy design.
Linking to Materials Science and Green Chemistry
Materials scientists like a reliable building block too. The same features that help in drug design support the fabrication of molecular electronics and smart polymers. Adding a carboxyethyl piece boosts solubility and helps the material attach to surfaces or other polymers. I’ve watched this compound at work while friends in material labs used it to graft molecules onto surfaces, boosting conductivity and function in electronic sensors or water filters.
Sustainability can’t stay off the table anymore. Using this reagent, teams can simplify routes that would otherwise involve harsh reagents or steps with toxic by-products. In my experience, that means less hazardous waste and lower costs over months or years. Students get to learn in a safer environment, and companies save money, which directly benefits everyone downstream—from researchers and manufacturers to patients and consumers.
What Comes Next?
Bringing 2-Carboxyethyltriphenylphosphonium Bromide into practical chemistry doesn’t fix every challenge. Some obstacles remain: sourcing high-purity material, ensuring reproducibility at scale, and training future scientists to use it safely. Industry and academia both have to stay sharp on safety practices and transparent data sharing. Facing these hurdles together, the community stands to push discovery forward, making tomorrow’s medicines and materials a bit more accessible and a lot more efficient.
Why Storage Matters for Specialized Chemicals
People in labs handle a lot of bottles, most with long chemical names and stories you can’t guess just from the label. 2-Carboxyethyltriphenylphosphonium Bromide is one of those that deserves extra attention. Doctors, researchers, and chemists all know that sloppy storage sets up risk—not just for ruined samples, but for ruined experiments or even serious health issues. Safety isn’t an afterthought; it’s fundamental.
Understanding What’s Inside the Bottle
Looking at the name, you realize this isn’t sugar for your coffee. This type of compound falls under the family of quaternary phosphonium salts. Most of these chemicals react poorly with high humidity or direct sunlight. I remember a professor telling us how water in the air can attack the chemical structure, making it lose that hard-won purity over weeks or months. If a lab tech uses it after moisture sneaks in, actual yields and results get skewed.
Practical Storage: Cool, Dry, and Out of the Sun
Most failures start simple: warm storerooms, leaky seals, bottles set too close to heat. My own time in the lab taught me that shelf placement matters just as much as the label. For 2-Carboxyethyltriphenylphosphonium Bromide, a cool room—stable, with temperatures around 2-8°C—keeps things steady. Opening the bottle in a humid or poorly ventilated space means you let moisture inside. Some labs use dedicated refrigerators with clear logbooks for sensitive chemicals. It’s not overkill. That discipline pays off.
Direct sunlight kicks up the risk. UV light doesn’t just fade labels; it cracks open chemical bonds, breaks down what you need for your reaction, and wastes money. Storing this compound in amber-colored bottles helps, but placing those bottles deep in the cabinet, away from the light, stacks the odds in your favor.
Safe Handling: Less Glamorous, More Essential
Spills and contamination usually come from bad habits, not bad luck. I learned from messy shelves, unmarked lids, and a few close calls on my own bench. Always check the lid after each use. Double-check your personal protective equipment—gloves, goggles, and lab coats. Don’t just clean the counter, clean the neck of the bottle too.
Label Everything, Track Every Move
No one wants to guess the age of their chemicals. The best labs run tight records, marking down every time the bottle is opened, logging the date, and listing who did it. It’s not just bureacracy. Logbooks give accountability and prevent confusion about chemical stability next time someone pulls that bottle from the back shelf.
Solutions: Keep Tools, Training, and Rules Up to Date
Supervisors and lab managers should run regular inventory checks, using electronic systems to flag older stock. Training isn’t a one-time deal. Newcomers and experienced staff both need hands-on refreshers. Updated procedures block careless mistakes. Simple rules—like never storing reactive chemicals near strong acids, bases, or solvents—protect everyone in the lab.
Protecting 2-Carboxyethyltriphenylphosphonium Bromide means respecting both the science and the risks. In real life, a disciplined system beats wishful thinking every time.
Looking Closer at the Chemistry
2-Carboxyethyltriphenylphosphonium bromide isn’t as famous as bleach or ammonia, but it turns up in labs dealing with organic synthesis. The questions about its safety pop up for a good reason. Many phosphonium salts, especially in a lab full of chemical bottles, raise eyebrows among both researchers and watchdogs.
Assessing Hazard Potential
Plenty of us have worked in settings where chemicals like this end up forgotten on dusty shelves. Sometimes folks treat these niche reagents with the same casual attitude as table salt just because their toxicity isn’t widely discussed. That’s a risky habit. Even when a chemical doesn’t have the flashing danger symbols pasted all over the bottle, it can still cause harm, especially if people ignore the basics—don’t eat near it, don’t touch your face, don’t go without gloves.
Not every phosphonium compound gets lumped together, but the group shares common risks. They often act as irritants, and 2-carboxyethyltriphenylphosphonium bromide lands squarely in that territory. It can irritate the skin and eyes. Breathing in the dust is nobody’s idea of a good day. There’s also a worry about long-term exposure. While animal studies on this exact compound aren’t all over the literature, folks in the lab pay attention to the structure: heavy aromatic rings, large cations, the halide counterion. Chemicals that look like this sometimes find ways to sneak through membranes in the body—and sometimes wind up more toxic than anyone expects.
Why This Matters Beyond the Lab
Anyone handling chemicals feels the pressure to never underestimate what’s in the bottle. In my early years working around synthesis labs, I saw how easy it was for a spill or poorly cleaned beaker to start a chain of accidents. Direct contact might only leave a red mark, but it takes only one inattentive moment to track unknown residues home or to contaminate shared workspaces. That’s something hard to explain if you’ve never seen coworkers scrambling for the eyewash station.
Most environmental agencies and regulatory bodies, including the European Chemicals Agency, approach new chemicals with caution. The law sometimes lags behind science. Just because no sweeping restriction exists for a reagent, that doesn’t mean it’s risk-free. Phosphonium salts can also be persistent in the environment and sometimes resist easy breakdown. Their impact on aquatic life remains an active question, but many similar compounds put a real load on wastewater treatment plants.
Practical Safety Measures
University labs and industrial workplaces rarely gamble with uncharacterized chemicals anymore, but safety improvements never happen fast enough. The best approach uses fume hoods, gloves, and eye protection. This isn’t just box-ticking for lab audits; it’s basic respect for your own health and your coworkers. Emergency protocols help, but day-to-day vigilance makes the difference.
People with years of lab time know that most incidents start with lapses in routine, like skipping gloves or mislabeling bottles. Good habits matter. Facilities should offer training that doesn’t just recite rules but actually shows why those steps protect everyone.
Building a Safer Chemical Culture
If you work with 2-carboxyethyltriphenylphosphonium bromide, keep updated with reliable safety data and product-specific risks. Sharing that information and experience with others saves trouble down the line. Labs can build a culture of care where nobody shrugs off irritating symptoms or ignores odd reactions during experiments. The best results happen where respect for people sits right next to progress in chemistry.
The Details Behind the Numbers
Saying “2-Carboxyethyltriphenylphosphonium Bromide” out loud might make even a seasoned chemist pause for a second. Behind this mouthful stands a specialized compound often found in organic synthesis or laboratory settings. The precise molecular weight of this compound is 444.32 g/mol. Knowing this number helps people working with it stay accurate in their measurements, which becomes crucial for reliable chemical reactions and predictable results.
Why Accurate Measurement Shapes Outcomes
Every bit of chemical work I’ve done comes down to the numbers. Skipping a decimal, picking the wrong molecular weight, or rushing the math can turn a simple experiment into a mess. Getting the molecular weight right for substances, even the exotic ones like 2-Carboxyethyltriphenylphosphonium Bromide, helps keep reactions balanced. One mistake multiplies downstream, affecting yield, purity, and even safety. In my younger lab days, a miscalculated molecular weight once left me with unexpected byproducts—and let’s just say the boss was not amused.
Connecting Molecular Weight to Real Work
In research, small details pack big punch. If someone scales up from a test tube to a liter flask, a wrong measurement could mean wasted money, lost time, or even dangerous side reactions. Each molecule brings its own quirks. In the case of 2-Carboxyethyltriphenylphosphonium Bromide, the correct number means the difference between a clean isolation and a lab bench headache. It’s about more than just math; precision sets the stage for both safety and curiosity-driven innovation.
Practical Tips and Reliable Checks
Relying just on memory or copying numbers from an old lab manual can spell disaster. Trusted chemical suppliers and peer-reviewed databases like PubChem or Sigma-Aldrich provide up-to-date molecular weights. Cross-referencing remains a regular part of my work. Following the rule, “trust but verify,” is what has saved my results more times than I’d like to admit. Double-checking molecular formulas, looking for recent data, and working with molecules in small batches until confident in the math keeps the work accurate.
Beyond Numbers: Safety and Responsibility
The weight of a molecule guides how much to measure, but the responsibility goes deeper. Handling chemical reagents requires respect for both the substance and those around you. The right calculation contributes to a safer workplace, protects expensive investments in reagents, and cuts down on unnecessary disposal or environmental risks. Many researchers have found themselves needing to answer hard questions about a waste stream or contamination, and the root usually traces back to an overlooked detail somewhere—in many cases, the molecular weight.
Building Trust Through Transparency and Accuracy
The relentless focus on details—starting with something as simple as checking the molecular weight—builds trust in results. That simple act gives colleagues, readers, and collaborators reason to believe in published data. In fields where trust is earned by years of careful work and could be lost with a single careless error, keeping things transparent, citing credible sources, and working through calculations in detail set the tone for solid science. This isn’t about overthinking; it’s about shaping habits that protect both reputations and futures in any research setting.