2-Hydroxy-N,N,N-Trimethylethanaminium Acetate: Deep-Dive Analysis
Historical Development
Long before 2-Hydroxy-N,N,N-Trimethylethanaminium acetate found its way into modern laboratories and processing plants, quaternary ammonium compounds like it began reshaping organic synthesis and industrial chemistry. Early studies explored their unique ionic character, particularly their dual nature—hydrophilic and hydrophobic. Chemists in the mid-20th century recognized the utility of choline derivatives for catalysis and phase transfer reactions. As research in green chemistry evolved, these salts caught more interest for their low volatility and promising safety benefits, causing a shift away from volatile organic solvents. From pharmaceutical formulation in the 1970s to innovative solvent systems in recent decades, the trajectory reflects increasing trust and reliance on compounds balancing reactivity with manageable safety profiles. The growing momentum behind sustainable chemistry only accelerated investigations into this molecule.
Product Overview
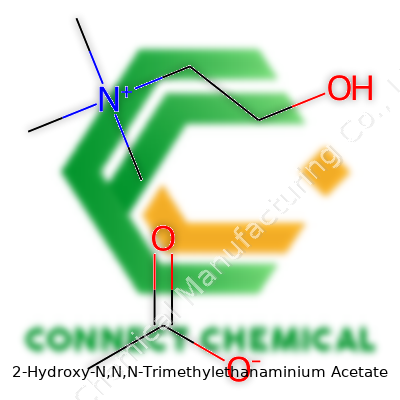
2-Hydroxy-N,N,N-Trimethylethanaminium acetate, known in many labs as choline acetate, sits among the simple yet highly functional ionic organic salts. Common sources supply this compound as a crystalline solid or aqueous solution. Its attractively simple molecular framework hides a host of industrial roles, mostly owed to how effortlessly it complexes with water and how readily its ions interact with a diverse spectrum of organic and inorganic materials. Products based on this salt support manufacturing workflows ranging from catalysis to biochemistry, often chosen where environmental impact matters as much as technical performance. The ready solubility, thermal stability, and non-hazardous reputation of choline acetate elevate its practical value across fields that struggle with traditional solvent toxicity and process difficulties.
Physical & Chemical Properties
Assessment by fresh eyes in an industrial lab reveals a white, hygroscopic powder or a transparent, nearly odorless liquid when dissolved in water. The molecular weight sits near 165.22 g/mol, providing a convenient handle for formulation scientists who design by stoichiometry. Its melting point typically lands below 110°C, and its good miscibility with water, methanol, and ethanol widens application options. Strong ionic interactions anchor this molecule in aqueous and some organic media, endowing it with a high dielectric constant and low vapor pressure—an important point for minimizing inhalation risks. The acetate counterion supplies buffer capacity in synthetic protocols and fermentation setups that demand pH stability. Somebody might shrug at these data points, but anyone who runs a crystallization or manages scale-up processes values how this compound’s nonvolatile and deliquescent character smooths out operational headaches.
Technical Specifications & Labeling
Standard product specifications report purity levels often surpassing 99%, moisture content below 1%, and heavy metal limits measured in parts per million. Analytical profiles should include clear batch traceability, which many purchasing managers emphasize after recalls or legal skirmishes draw attention to gaps in supply chain documentation. Labels must specify the product’s chemical name, CAS number, physical form, and storage instructions, alongside hazard warnings and regulatory classifications. Specific data like pH of a 1% solution, refractive index, and residual solvent content land on technical data sheets, targeting downstream users who require batch-to-batch consistency. While regulatory designations remain modest compared to more reactive amines and quaternaries, compliance with the latest GHS and transport guidelines earns trust from safety officers and regulatory auditors.
Preparation Method
Production of 2-Hydroxy-N,N,N-Trimethylethanaminium acetate generally follows a straightforward acid-base neutralization. Industrial operators introduce high-purity choline hydroxide to glacial acetic acid under controlled aqueous conditions, keeping temperatures below 40°C to avoid decomposition. After neutralization, vacuum drying or solvent evaporation removes excess water, sometimes followed by recrystallization when pharmaceutical or electronic grade material is required. Many plants now track real-time pH and conductivity in these syntheses, ensuring no excess base or acid carries forward to finished product. Downstream filtration and packaging in moisture-barrier containers wrap up the process. Automation, remote monitoring, and waste minimization reflect priorities in today's chemical manufacturing, because the call for greener, more efficient processes echoes across every sector.
Chemical Reactions & Modifications
Choline acetate steps into chemical reactions as a phase transfer catalyst, biocatalytic enhancer, or mild buffer. Its quaternary ammonium group enables phase separation in multiphase systems, letting it shuttle anionic or cationic species across boundaries other catalysts ignore. The acetate side brings hydrogen-bond accepting abilities, which subtly steer non-covalent interactions in organocatalysis, polymerization, and enzyme stabilization. Derivatization builds upon the N,N,N-trimethylethanaminium core; chemists have swapped out the acetate for formate, chloride, or even longer-chain fatty acids, tuning hydrophobicity and biodegradability for different purposes. Thermal decomposition or reaction with strong acids can generate methylated side-products, so experienced hands watch closely for off-spec smells or discoloration. These features create a clutch player in academic labs looking for clean, simple transformations and in factories chasing tighter environmental controls.
Synonyms & Product Names
Chemists often call 2-Hydroxy-N,N,N-Trimethylethanaminium acetate by its colloquial name, choline acetate, saving a few breaths during team meetings. The salt carries a host of synonyms in regulatory filings and supplier catalogs, including N,N,N-Trimethyl-2-hydroxyethanaminium acetate, Trimethyl(2-hydroxyethyl)ammonium acetate, or even Choline ethanate among others. Trade names sometimes bundle relevant specifications or intended uses, especially from companies offering pharmaceutical or high-purity grades. These alternative names gain relevance in multi-lingual regions or when cross-referencing international patents and regulatory lists. Anyone searching academic literature, patent repositories, or import-export databases learns to keep an eye out for less obvious synonyms, cutting off supply chain headaches or research duplication at the root.
Safety & Operational Standards
Material safety data leaves little doubt: 2-Hydroxy-N,N,N-Trimethylethanaminium acetate ranks among safer quaternary salts. Most safety tickets cite low acute toxicity and minimal risk under proper handling, though skin and eye contact still call for the usual gloves and goggles. Dust generation may pose mild respiratory irritation, so lab personnel often use powder hoods or local exhaust. On-site spill clean-up relies on standard PPE, scoop, and water rinse—no dramatic measures required. The compound skips most flammability ratings and remains stable away from high heat or strong oxidizers. Regulatory status sits comfortably between food additive choline salts and more complex amine-based process chemicals, meaning waste handling and effluent discharge often dovetail with broader chemical management policies. Regular workplace training and review of local environmental release thresholds ensure a high safety baseline.
Application Area
Fieldwork by chemical engineers and process specialists points to a spectrum of uses for choline acetate. In biochemistry and molecular biology labs, it supports buffer systems for cell culture and enzyme assays, thanks to its mild reactivity and biocompatibility. Plant extractors and flavor formulators use it as a water-miscible co-solvent, extracting target molecules without denaturing sensitive organics. The pharmaceutical sector exploits its solubilizing and stabilizing traits in drug delivery systems, especially for hygroscopic or fragile actives. Green chemistry projects turn to this salt as a non-toxic alternative to traditional solvents, where its biodegradability and low vapor pressure clear regulatory hurdles. Battery and electronics manufacturers test its ionic fluid properties for safe, sustainable energy storage. Veterinary and medical researchers keep it on hand for nitrate reduction and microbial culture networking. Wide acceptance in feed manufacturing and functional foods follows from its choline backbone and recognized nutritional value. Each sector—industry, academia, and public health—brings its own priorities, but all pay attention to the combined safety, availability, and technical versatility it offers.
Research & Development
Scientists pushing boundaries keep 2-Hydroxy-N,N,N-Trimethylethanaminium acetate under the microscope for everything from new solvent systems to enzyme engineering. Teams in universities and private R&D hubs lean on its ability to stabilize proteins, improve phase transfer rates, and provide a nontoxic ionic environment. Ongoing projects test it as a carrier for smart drug formulations and as a component in room-temperature ionic liquids—promising new classes of solvents for selective organic reactions. Recent breakthroughs explore bio-based synthesis routes using fermentation by-product streams, aiming to slash petrochemical feedstock reliance. Many analytical chemists study its interactions in multi-component buffers, predicting outcomes and troubleshooting reactions faster than ever before. Funding authorities recognize these efforts, seeing the sustainability and safety advantages that could ripple across pharmaceuticals, food, and green technology industries.
Toxicity Research
Animal and ecosystem studies rank 2-Hydroxy-N,N,N-Trimethylethanaminium acetate low on the toxicity ladder. Acute oral and dermal toxicity rates remain mild, with large LD50 values showing little risk to rodents in controlled environments. Longer term studies in aquatic organisms highlight rapid biodegradation and little impact on reproduction or food chain dynamics, a refreshing contrast to the persistence problems that cloud more complex quaternary salts. Human occupational exposure measurements in production settings rarely indicate significant health risks, with good engineering controls and PPE accounting for the residual odds. Researchers still watch for subtle effects, such as minor biochemical shifts at very high doses or chronic ingestion, but so far findings reinforce the safety message most regulatory bodies convey. In regions with tougher environmental release laws, those traits earn it a place at the table for new product development and green infrastructure planning.
Future Prospects
Change keeps pace with technology in the world of functional chemicals, and 2-Hydroxy-N,N,N-Trimethylethanaminium acetate stands out for its adaptability and trustworthy record. Chemists, biologists, and process engineers experiment with bio-based production, circular chemistry models, and custom salt derivatives for ever more specific performance tuning. Electronics manufacturers and battery innovators assess its use in solid-state and hybrid electrolyte systems geared for lower toxicity and higher resource efficiency. Pharmaceutical and food technology sectors move toward more biocompatible excipients and stabilizers, pushing this sort of salt higher up the preference list for regulators and R&D strategists alike. Policy trends set the scene for stronger pushback against legacy solvents and less degradable ionic species; these currents open the door wider for choline acetate and similar compounds. As laboratories and production floors embrace more digital workflows and tighter standards, the future value of chemicals that blend safety, performance, and sustainability seems set to grow.
The Substance and Its Everyday Relevance
In the world of chemistry, some names sound so complicated they almost push us away. 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate stands as a tongue-twister for sure, but a closer look reveals it plays a useful part in real-life applications you probably never noticed.
Lifting the Fog on its Purpose
Most people know this compound under its more approachable name: Choline Acetate. Choline is an essential nutrient. Our bodies need it for basic cellular function, nerve signaling, and keeping our liver healthy. What makes 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate stand out in its acetate salt form is its role as a supplement and a building block in science and technology circles.
Packing a Punch in Nutritional Science
Choline acetate doesn’t show up in candy bars or soda cans, but it can pop up as a supplement. Diets low in choline can lead to problems with memory, muscle control, and even liver health. Scientists learned early on that acetylcholine—a neurotransmitter vital for memory and muscle movement—relies on steady choline supplies. Some clinicians suggest choline acetate to folks with a rare genetic inability to build choline molecules on their own. In practice, nutritional supplements featuring this compound usually target those looking for better cognitive function or those battling certain liver disorders.
Beyond the Human Body: Industrial Chemistry Uses
Industrially, 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate isn’t just about brainpower. Researchers often call it an ionic liquid, which means it can work as a “green” solvent in chemical labs. During the past decade, the world of chemical processing has started moving away from harsh solvents toward greener options. Choline acetate solves a problem here—it dissolves a wide range of compounds, helps speed up reactions, and, unlike many alternatives, breaks down easily without sticking around to harm plants or animals.
Scientists lean on this compound in areas like biomass processing, drug formulation, and biodegradable plastics. Some labs use it to pull beneficial chemicals out of plant material—coffee husks, wood fiber, and other leftovers. Others rely on it to help separate valuable pharmaceutical ingredients from impurities.
Potential Risks and Responsible Use
Anything that lands in food supplements and industry deserves careful oversight. Choline compounds in high doses can trigger side effects: fishy body odor, low blood pressure, sweating. That’s why nutrition experts pay close attention to suggested daily intakes. In industry settings, the record looks clean; choline acetate’s high biodegradability and low toxicity give it a friendlier profile compared to other chemicals.
Pushing for Sustainable Solutions
I remember talking to university researchers eager to cut waste from biofuel production. They shared case studies using choline acetate as a solvent for plant fibers. Compared to petroleum-based solvents, it performed with less environmental impact and delivered fast results. If more folks in manufacturing shifted toward greener solvents like choline acetate, the world could lower chemical waste and gain higher recovery from renewable materials.
A compound with such a complex name ends up connecting nutrition, advanced chemistry, and sustainability. That’s worth remembering, not just for scientists, but for anyone curious about the natural world and how we shape it.
Conditions Matter More Than You Think
Storing products correctly doesn’t just maintain value; it keeps people safe and avoids expensive surprises. Having spent a decade in supply chain roles, I’ve come across a long list of products that demanded more than a shelf and a label. Around food, medicine, or chemicals, storage conditions get personal. Consider temperature. In hot climates, a warehouse can feel like an oven. Leaving cartons in the wrong spot can ruin stock and hurt people who later use it. Refrigerated goods demand reliable cooling, with alarms and daily checks, not because companies like rules, but because effectiveness can vanish after one bad day. Lapses mostly happen from ignoring daily routines or cutting corners during a busy week.
Light exposure causes trouble few suspect. We once lost an entire shipment of vitamins because the loading bay had skylights—letting in just enough sunlight to degrade the batch. Labels clearly said, "store away from direct light," but someone unfamiliar with the risks gave them prime real estate near those windows. Some packaging breaks down with light, which lets air and moisture creep in. The lesson sticks with you after watching a manager explain why two months of stock went in the trash.
Moisture, Air, and Cleanliness: The Hidden Factors
Humidity sneaks up too. Moisture clings to packaging, seeps into powders, and fuels mold where you least expect. Years ago, I visited a warehouse in the rainy season and noticed condensation dripping from the ceiling. After a week, flour stored nearby clumped up and failed quality checks. Every warehouse I managed since then got dehumidifiers in the rainy months and regular maintenance on the roof. Above all, keep the floor dry—once moisture forms puddles, rodents and insects show up.
Cross-contamination makes headlines for deadly reasons. In food and drug storage, segregate strong-smelling products (think spices, chemicals) from everything else. I recall a shipment of cleaning agents stacked beside tea. Both products arrived sealed, but the tea carried a faint chemical odor by the time it reached stores. Customers noticed, and we ate the losses. Keeping products in their own spaces, on proper pallets, well away from walls, saves more than just money. It keeps trust intact.
Access, Records, and Training
Safeguarding inventory isn’t just about physical conditions. Access control keeps people honest and goods secure. Not once, but several times, I’ve seen staff grab what’s easiest to reach instead of what’s oldest, leading to expired products on the shelf. A robust inventory system keeps track of every lot, flagging soon-to-expire batches, and shows exactly what entered and left the warehouse. Technology helps, but vigilance and simple training do most of the heavy lifting.
Labels tell most of the story if people read them. Manufacturers spend money testing storage conditions, designing packages, and writing clear instructions. As a manager, I made sure staff could identify danger warnings, and understood the importance of the storage temperatures listed. In my experience, refresher sessions every few months save headaches later.
Improving Storage Practices
Improvement begins with honest risk assessment. Check insulation, measure air flow, and keep humidity logs. Regular audits expose bad habits before they become disasters. Invest in training and treat warehouse teams as safety leaders. In my career, building a team invested in doing things right delivered better results than any top-down rule.
Getting to the Heart of the Matter
People sometimes scroll past the chemical name—2-Hydroxy-N,N,N-Trimethylethanaminium Acetate—faster than they'd scan a privacy policy. This compound, more commonly connected to the choline family (yes, as in choline vitamin supplements), appears in labs and a handful of industrial settings. But the question keeps popping up: Just how risky is it to work with or come into contact with this stuff?
Looking at What’s Known
Choline salts like this one matter in many biochemistry settings, but not everything that sounds “chemical” means trouble. Genuine worry should steer toward facts: what does science know about its toxicity or potential hazards? Safety data sheets from major suppliers flag 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate as a substance with low acute toxicity. Unlike chemicals that carry skull-and-crossbones warnings, this one often lands in the “irritant” category, not the “hazardous” zone.
Research shows most choline derivatives don't build up in the body and flush out without much fuss. A look at accidental ingestion studies tells the same story. There’s no sharp jump in hospital visits linked to this compound, and regulators have not given it a “hazardous” rating for consumers or workers. With the facts in hand, it looks milder than a lot of the cleaning products you keep under the kitchen sink.
Understanding Exposure Risks
Even so, nothing deserves blind trust. Lab techs learn early on to avoid inhaling powders or getting chemicals in their eyes, even with compounds that look safe on paper. Local irritation—red skin, itchy eyes, maybe a sneeze attack—can flare up for folks handling any salt or acid, including this one. Gloves, goggles, and a quick rinse can stop discomfort before it starts.
Allergies and rare sensitivity come with every workplace. Direct, repeated rubbing against skin, or breathing in a dusty roomful, could create irritating effects. But that’s true for table salt and flour as well. My years in biochemistry labs taught a simple lesson: If something splashed on my arm made me tingly or red, the best fix was soap, water, and a moment at the eyewash station. Choline acetate never made the “danger zone” list for us, but respect kept us from skipping protection.
Environmental and Broader Health Questions
Now, some people wonder about environmental run-off or what happens if large amounts hit the waste stream. With choline being an essential nutrient and acetate turning up in vinegar, there’s little evidence this combo triggers ecosystem disasters. Regulatory agencies leave this chemical off their red-flag lists for water or soil safety.
Making Common-Sense Choices
Just because something avoids “high hazard” status doesn’t give anyone a free pass. Spills need clean-up, storage wants a closed lid, and nobody should drink from lab bottles. Teachers, workers, and hobby chemists need updated safety sheets and a healthy respect for any chemical. Check your gloves for holes and keep snacks far from the workbench—habits that kept my colleagues and me out of the emergency room for years.
In short, 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate does not show signs of significant risk in small-scale, managed use. That doesn’t mean it belongs in open drinks or playrooms. With the right information and habits, people can work with it safely, without extra stress or fear. Real hazard starts when caution takes a back seat.
Everyday Toxicity Hiding in Plain Sight
Most homes collect more hazardous stuff than you’d expect. Batteries roll around in kitchen drawers, cleaning sprays gather under sinks, and half-empty paint cans sit forgotten in the garage. Tossing these into the regular trash feels tempting, but the cost to health and the planet snowballs fast. Landfills don’t handle chemical leaks well. Nobody wants groundwater laced with bleach or forests tainted with lead. I’ve seen neighborhood recycling days fill up with items that remind us—out of sight isn’t out of harm.
Health Risks and Real Consequences
Dumping old batteries or medications in the trash seems quick. Still, toxins slip out and join rainwater, seeping into streams and soaking into soil. Studies link mercury from tossed bulbs and batteries to nerve damage in kids. Medicines flushed down the toilet pass through water treatment, then flow into rivers, messing with fish hormones and even turning up in drinking supplies. Parents want safe playgrounds and clean tap water. Safe disposal offers a direct way to protect both.
Local Solutions and Community Responsibility
Any town with a public works department likely schedules hazardous waste drop-off events. I remember dragging a box of junk from my garage—old motor oil, dead phones, dried-up paint—to a parking lot and seeing a steady line of families relieved to part with their unwanted stuff responsibly. Cities keep lists online of where to ditch specific products. Grocery stores often collect used plastic bags and expired batteries up front. Medicines go back to pharmacies offering take-back bins.
All it takes is a quick search or asking a clerk. When I hear about people sneaking things into the trash, I point out how easy collection has gotten. Many local governments fund these services because the long-term harm from improper disposal costs communities so much more.
Simple Habits for Less Trouble
Product labels offer disposal tips, and looking up a symbol or recycling number never takes long. For paint, letting leftover liquid solidify before tossing helps, but oil-based cans belong at hazardous drop-offs. Unused meds should never end up down the drain. Instead, mix them with coffee grounds or kitty litter if take-back isn’t available, then put them in a sealed bag. Old phones and batteries often get re-used for parts or raw material, but putting them in the right box at the electronics store brings more second chances.
Teaching kids early helps too. At home, we keep a box where spent batteries or broken bulbs gather, then empty it together every few months. Turning safe disposal into a normal chore sets a pattern that sticks.
Avoiding Toxic Loops: Everyone Plays a Role
Each household nudges the environment one direction or another. It doesn’t take a regulation to change the routine—just a nudge and a little accountability. Just as sorting plastics or rinsing cans turned into everyday habits, treating hazardous materials carefully folds into the rhythm when the path is clear. Community drop-offs, honest labeling, and a willingness to look up disposal sites connect everyone’s action with bigger health for families, water, and air.
Disposing safely protects more than just a single bin or a patch of ground. It keeps harm from circling back down the line. Facts, not just rules, show what can go wrong, but real progress comes from making the right choice easier for everyone. That’s something every home can aim for—and it never sits idle waiting for someone else to do it.
The Chemistry at a Glance
Every molecule tells a story, and 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate delivers on that. Its chemical formula is C7H17NO3. This compound features a quaternary ammonium backbone with an acetate counterion. Piecing it together isn’t rocket science, it just takes a little patience and some attention to detail.
The molecular weight sits at 163.22 g/mol. Calculating this involves adding up contributions from each atom: carbon at 12, hydrogen at 1, nitrogen at 14, and oxygen at 16. These numbers aren’t mysterious—they come straight from the periodic table most of us memorized in high school. This value is more than just trivia for chemists. It’s a number that guides real-world actions, from accurate dosing to predicting behaviors in a mixture.
Why This Matters in Real Life
This molecule isn’t just a textbook example. It pops up in biochemistry labs and industrial settings. Researchers count on the precision of its molecular weight during synthesis or when running chromatography. I've watched students get tripped up by using the wrong weight, throwing an experiment off course. Having the right numbers upfront saves a lot of headache later on.
Beyond the lab, the structure of this molecule offers clues about its strengths and weaknesses. The trimethylammonium group helps draw in water and other polar solvents. That means companies count on it for tasks that need a bit of chemical persuasion, whether it's separating components, altering pH, or acting as a buffer. It balances efficacy with gentleness toward biological systems, an edge that attracts researchers in pharmaceuticals and life sciences.
Facts to Lean On
This molecule stems from choline derivatives and plays a role as a building block in many biological systems. Its backbone links directly to choline, which bodies rely on for brain function, among other things. Companies in the supplement and pharmaceutical business take notice of molecules like this when developing new products. Widespread databases such as PubChem and ChemSpider list this compound with the formula C7H17NO3 and molecular weight 163.22 g/mol, so there’s clear consensus on its makeup.
Safety guidelines reinforce why detailed knowledge about molecular weight and formula proves critical. Overdosing, even by accident, can shift a compound from being helpful to harmful. In the real world, that translates to real stakes for researchers and technicians. I remember watching a colleague recover an experiment, only after realizing his solution relied on the wrong molecular mass and had to be remade from scratch. That kind of setback eats into timelines and budgets, a reminder that chemistry is as much about diligence as it is about discovery.
Solving Real Problems with Chemistry
Greater transparency goes a long way. Reliable chemical supply chains and accurate product sheets lower the odds of mistakes that start with a misreading of formula or molecular weight. Education matters, too—I’ve spent years in classrooms stressing the need to double-check calculations before heading to the bench. Online calculators and reference materials help level the playing field for both novices and veterans. Industry bodies can foster better cross-checks and better documentation to reinforce habits that prevent these basic errors from mushrooming into much bigger problems down the line.
As science moves fast, taking time for accuracy never gets old. 2-Hydroxy-N,N,N-Trimethylethanaminium Acetate shows how one molecule can bring lessons in both structure and responsibility, if folks take the time to look.