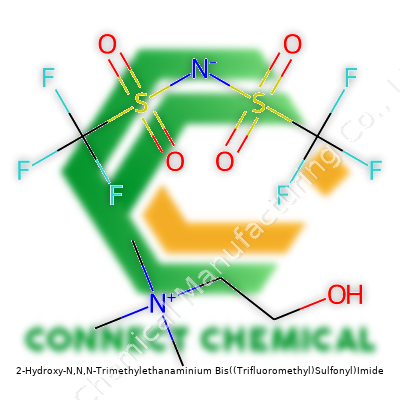
2-Hydroxy-N,N,N-Trimethylethanaminium Bis((Trifluoromethyl)Sulfonyl)Imide: Beyond the Formula
Historical Development
Curiosity about new solvents grew fast after the 1980s, especially as researchers looked for alternatives that worked without the usual toxicity and flammability. Ionic liquids started to get real traction in laboratories that needed tough, reliable, non-volatile materials. 2-Hydroxy-N,N,N-Trimethylethanaminium bis((trifluoromethyl)sulfonyl)imide—often shortened for sanity as Choline-TFSI—emerged from a wave of work on “task-specific” ionic liquids. Chemists pooled their ingenuity to tweak molecular structures, and choline-based ionic liquids drew attention thanks to the natural abundance of choline. Choline hits the right notes for bio-based sourcing, and pairing it with strong, hydrophobic anions like TFSI took these early concepts out of the flask and into industries hunting for performance with less hazard.
Product Overview
This compound stands as a member of the choline salt family, marked by a hydroxylated quaternary ammonium cation and the mighty bis(trifluoromethylsulfonyl)imide anion. It appears as a colorless or pale viscous liquid, staying pleasantly stable under ordinary conditions. Users appreciate its complete miscibility with water, a trait rare among ionic liquids containing large, fluorous anions. Laboratories draw on its unique profile for green chemistry, electrochemistry, and even the trickier demands of materials processing. The robust chemical structure promises versatility far beyond simple salt replacements.
Physical & Chemical Properties
Choline-TFSI offers a density around 1.3-1.4 g/cm3 at room temperature. As a liquid at ambient conditions, it avoids the historic headache of crystalline residue or drying necessity. Thermal stability takes it up to around 250-300°C before decomposition—impressive for an organic-based liquid. Viscosity depends on purity and temperature, tending toward a heavy oil feel that thins out as heat increases. The ionic character leads to high conductivity, easily reaching several millisiemens per centimeter, a vital property in battery and supercapacitor electrodes. Unlike classic organic solvents, its vapor pressure remains extremely low, so accidental inhalation exposure drops off the risk list.
Technical Specifications & Labeling
Suppliers typically ensure a purity greater than 99%, required water content under 0.1%, and low trace metal contamination. Labels often provide batch number, date of manufacture, and handling recommendations. Chemical identifiers include the CAS number 79922-15-1 and formula C8H18F6NO5S2. Shipping containers vary from amber glass to high-density polyethylene based on volume, with rigid seals to keep out atmospheric moisture.
Preparation Method
Preparation starts by reacting choline hydroxide (or chloride, sometimes) with lithium bis(trifluoromethylsulfonyl)imide in an aqueous medium. Metathesis precipitates lithium chloride, which can be filtered away, freeing the desired ionic liquid in solution. Rotary evaporation removes bulk water, and further drying under reduced pressure ensures a product fit for demanding electrochemical applications. The process avoids chlorinated solvents, sidestepping many older environmental headaches.
Chemical Reactions & Modifications
The hydroxyl group on the choline moiety serves as a handy anchor for further modifications. Researchers graft polymer chains, cross-linkers, or even attach metal centers, creating hybrid materials tuned for catalysis, membrane formation, or selective extraction. The TFSI anion’s stability deters most unwanted side reactions, so performance doesn’t drop across a range of redox chemistries. Some work explores swapping the TFSI for other fluoro-sulfonyl counterparts, each with trade-offs in hydrophobicity or electrochemical stability.
Synonyms & Product Names
Commonly listed names include “Choline TFSI”, “Choline Bis(trifluoromethanesulfonyl)imide”, “Choline TFMSI”, and “2-Hydroxyethyltrimethylammonium bis(trifluoromethylsulfonyl)imide”. Marketing sometimes uses “bio-ionic liquid” or “green choline salt” to reflect its partial roots in natural starting materials.
Safety & Operational Standards
Even though choline forms a vitamin in the human diet, TFSI puts a different spin on safety. Skin and eye contact deserve attention, so gloves, lab coats, and chemical splash goggles all earn their place on the workbench. Inhalation risks remain low from negligible volatility, but researchers using the powder or in confined spaces favor local extraction. No evidence currently links this compound with acute toxicity or environmental persistence like traditional chlorinated solvents. Still, disposal routes funnel waste toward incineration at licensed sites, not down the drain, showing respect for both safety and responsible stewardship.
Application Area
In energy storage, Choline-TFSI sees use in lithium-ion and sodium-ion battery electrolytes where non-flammable, ion-conducting media outpace older carbonate solvents. Supercapacitor researchers rely on its thermal stability and low environmental footprint. As a solvent, it dissolves cellulose without fuss, making it relevant for next-generation fiber spinning and biomass fractionation. The pharmaceutical industry checks out its potential for “deep eutectic” mixture formation, offering greener routes for drug synthesis and formulation. Labs working on carbon capture examine its CO2 solubility, looking for clues on how to bring emission controls forward without further chemical burden.
Research & Development
Papers keep rolling off the press as researchers tweak structures, test new blends, and benchmark performance against classic ionic liquids. Universities partner with energy consortia, eager to prove real-world stability for grid storage applications. Chemists try to graft functional groups onto the choline backbone and look for custom properties—catalytic, selective, biodegradable—that push the compound into new application fields. Analytical teams chase after new separation processes, keen on cost-effective recovery and reuse strategies, and industry giants keep a close eye on scaling production from the 10-gram to 100-kilogram mark.
Toxicity Research
Long-term studies point to low acute toxicity, although TFSI’s presence demands caution. In vitro work with model organisms reveals little evidence of bioaccumulation or mutagenicity. Regulatory focus stays sharp, especially in European markets where REACH rules encourage expanded toxicity tests before commercialization. Animal studies show rapid excretion rather than storage in fatty tissues, marking a sharp difference from older perfluorochemical hazards. Environmental fate largely follows pathways of hydrolysis and degradation, supporting the hope of a cleaner profile. Still, prudent management of spills and residues holds up under scrutiny.
Future Prospects
Demand for safer, high-performance solvents keeps rising as the world turns toward tighter sustainability targets and improved worker safety. Choline-TFSI template compounds look set to gain ground in energy storage and green manufacturing as technologists refine scale-up and purification. Design moves toward recyclable blends and expanded use in smart materials. When researchers unlock further cost reductions, this ionic liquid could step out of laboratory stockrooms and into feedstocks for larger-volume manufacturing, opening doors for greener electronics, textiles, and even specialty agriculture.
Stepping Into the World of Ionic Liquids
Ask anyone who worked in a modern chemistry research lab over the last decade about 2-Hydroxy-N,N,N-Trimethylethanaminium Bis((Trifluoromethyl)Sulfonyl)Imide, and you’ll likely hear three simple words: “That’s an ionic liquid.” Chemists appreciate these substances for how they upend traditional rules of solvents. Unlike water or oil, this liquid, often called Choline-TFSI for short, doesn’t evaporate easily, doesn’t catch fire under normal circumstances, and can dissolve both salts and organic materials with ease.
Far Beyond the Lab: Real-World Applications
Most typical solvents carry big risks in production plants. They smell, they burn, and they can pollute water. With Choline-TFSI, industries find fewer headaches. Looking at batteries, for example, lithium-ion versions need liquid electrolytes. These often react poorly with air or moisture, contributing to accidents and shortened device lifespans. Choline-TFSI stands out because it lets ions move quickly between electrodes, but doesn’t catch fire and forms a stable interface with common battery materials. Companies tinkering with next-generation phone batteries or grid storage feel attracted to this level of safety and flexibility.
Supporting a Greener Chemical Industry
My time in sustainable chemistry always returned to the question, “Can we swap out old polluting processes for something better?” Ionic liquids like Choline-TFSI make it possible. It doesn’t escape into the air during production. It resists breaking down under heat. During extractions or separations, labs use this material to separate rare earth metals, recycle valuable elements from electronics waste, or even pull out bioactive compounds from plants without making piles of dangerous waste. The environmental angle isn’t just hype. The choline part of this molecule comes from biomass—think corn or sugar beets—so it’s not only safer but also renewable. Trifluoromethylsulfonyl imide, on the other hand, gives the liquid its stability and broad chemical compatibility.
Challenges Waiting for Solutions
Chemists see promise, but a few hurdles keep popping up. Making Choline-TFSI pure enough for sensitive applications still costs time and money. Factories face high up-front investments in purifying and recycling the material itself. Fluorinated chemicals also bring up worries over persistence in the environment, and communities living near manufacturing sites notice that these concerns rarely vanish on their own.
Researchers and engineers hope for regulations that push real recycling. Efforts to reclaim and reuse ionic liquids often come down to creative thinking. Some techniques turn to membrane filtration, others to distillation, or new absorbents that catch waste before it leaves the plant floor. People in the field test hybrid ionic liquids that hold onto the green benefits without creating tough-to-break-down waste.
Looking Forward With Purpose
In workspaces everywhere, we need to keep weighing risk, cost, and safety—especially as new ionic liquids like Choline-TFSI move from laboratory curiosity to tools for technology and sustainability. Factories changing their solvent lists feel growing public interest in green chemistry. I see investors asking for proof of safety and a way to trace chemicals from cradle to grave. As more demand lands for safe therapies, faster electronics, and less-polluting manufacturing, Choline-TFSI could keep finding new roles, as long as safety, cost, and disposal stay at the front of the conversation.
Why Safety Steps Deserve Attention
I’ve spent plenty of days around busy labs and noisy workshops. Factory floors, classrooms, even home garages—all sorts of places hold chemicals people barely glance at. One spill or careless whiff can flip an average afternoon into a trip to urgent care. Even simple products change into hazards once they get into the wrong eyes, lungs, or skin. The stories stick with me: someone skipping gloves, a coworker ignoring a mask, another guy doubling up on safety gear just because he “felt lucky.” It usually doesn’t come down to luck; it’s about habits and knowledge.
Get To Know What You’re Handling
Picking up a chemical without reading its label or peeking at the sheet with all the warnings never makes sense. That safety data sheet (SDS) tells you if a splash can burn through skin, if the fumes could make you woozy, if water washes it away or sets off a nasty reaction. One time the SDS for a metal cleaner actually said, in plain words, “This reacts violently with bleach.” Turns out, mixing those two creates a toxic gas strong enough to clear out a whole row of classrooms—and that wasn’t just fiction. The details in these sheets come from real accidents, not legal-speak or overcautious scientists.
Keep Barriers Between You and Trouble
Gloves, goggles, a sturdy pair of boots—they sound simple and sometimes feel awkward. Still, they turn into armor against splashes, fine powders, or fumes. Lab coats don’t look cool, I admit. They keep acid off your shirts and your skin. Respirators—actual fitted ones, not the single-use dust masks—block out dangerous air particles. Poor fit gives a false sense of security. I learned early that forgetting to check seals could leave you coughing even if you wore the right thing.
Stay Alert With Storage and Clean-Up
Stacking bottles in the wrong cupboard or mixing leftovers into the sink once caused headaches at a friend’s art studio. The right containers, marked clearly by names you can actually pronounce, avoid mix-ups. Leaving things unlabeled does not just slow down your own process—it puts everyone else at risk, too. Clean-up means more than sweeping—it involves ventilating the workspace, neutralizing spills the right way, and throwing out rags or gloves that soaked up something nasty.
Practice Respect Over Fear
I remember a mentor who said, “Chemicals don’t judge, they just react.” Fear keeps some people away from valuable experiments, but ignoring the risks brings only regret. Regular training and refreshers help keep routines honest. Even experts trip up when tired or distracted. People working together—for example, keeping each other in check on protocol—prevent shortcuts. Reading about incidents, keeping up with public health bulletins, and swapping stories with others in the field kept my own habits sharp.
Find Help and Keep Learning
If something feels off—smell, color, a stain on your hand—getting advice can change the outcome. Many companies, universities, and hospitals run free classes. The CDC and OSHA share real-world updates online, including accident reports that might sound boring but often save a lot of pain. No shame in asking someone seasoned before handling something new. Sharing knowledge builds the safety net for everyone.
Understanding Product Stability
Most folks who work around chemicals, foods, or medicines learn pretty quickly that the way you store things makes all the difference. I remember the first time I worked at a pharmacy, the manager showed me a set of strict rules for keeping pills dry, cool, and out of sunlight. Later on, working in a restaurant, I saw similar lists for cheese and produce. Heat, moisture, and light cause trouble for a huge range of products, wrecking the ingredients or changing the way they work.
Heat and Light: Common Enemies
Leaving bottles or packaged goods on a sunny shelf usually cuts shelf life by a chunk. Studies show that most prescription drugs start breaking down after a few days if left above room temperature—think of insulin or antibiotics. Chemical products like adhesives or paints can thicken, clump up, or lose their punch after sitting in heat or sunlight, even for a short time.
Sunlight isn’t just bright. UV rays speed up breakdowns in some vitamins, turning hard-earned nutrients in cereal or multivitamins into near useless fillers. Most of the time, manufacturers put products in brown, blue, or opaque bottles for good reason. That little extra effort keeps the product doing its job for far longer, cutting down on waste.
Controlling Moisture
Water vapor in the air causes problems nobody wants. Powders turn to lumps. Capsules may stick together. Calcium chloride, found in basic dust-control or ice-melt bags, pulls moisture right out of the air if left open, eventually forming a solid lump that doesn’t work at all. Folks running warehouses learn quickly—seal those bags tight, and add desiccant packs when needed.
Even coffee, flour, or spices lose their punch if left open on the counter. Humidity creeps in and changes texture or flavor. I saw it firsthand watching a bakery turn out flat, tasteless cookies after someone moved flour from a cool storeroom to a shelf right above the dishwasher.
Following Clear Labels
Most products carry storage directions for a reason. “Store below 25°C.” “Keep in a cool, dry place.” These rules come from months of stability tests, often required by government agencies. Regulatory bodies like the FDA and EMA want proof that products stay safe and effective until at least the labeled expiry date. Failing to respect recommended conditions can quickly void manufacturer guarantees, and nobody wants to waste money tossing expired stock.
Smart Solutions: Simpler Than You Think
It doesn’t always take a fancy walk-in cooler to get things right. A closed cupboard away from heat vents or windows can keep most pills, vitamins, and grains stable for months more. Better yet, keeping products in their original packaging helps, since bottles and cartons are designed to keep out light and moisture. Instead of transferring goods into clear glass jars or plastic bins, stick with what the maker provided unless there’s a solid reason to switch.
Throwing a few extra dollars at temperature and humidity monitors for larger home pantries or warehouses pays back in lost savings. Even a cheap thermometer gives early warnings about risks. In industrial settings, regular shelf inspections catch leaks, torn packaging, or early signs of spoilage. Teams that make storage a habit have fewer headaches and less waste at the end of the year.
Why It All Adds Up
Proper storage stands at the foundation of product safety and effectiveness. I’ve seen both patients and businesses lose out by ignoring straightforward instructions. A few smart choices help keep the value and purpose locked in, serving those who need it without surprises. With attention and care, it takes little effort to preserve stability and stretch value further.
Understanding Purity Standards
In lab days, I remember opening chemicals with a hopeful glance at the label—99%, 95%, 98%, different numbers, but every single one meant something deeper than just “good enough.” For 2-Hydroxy-N,N,N-Trimethylethanaminium Bis((Trifluoromethyl)Sulfonyl)Imide, precision takes on meaning beyond comfort. Typical supplies of this ionic liquid reach 98% or above, often stretching to 99%+ in specialty chemical catalogs. Analytical verification, like NMR, HPLC, or elemental analysis, backs that number up, not just trust. Lower levels show up here and there, but high-purity is the rule across battery, catalysis, and separation labs.
Impurities, even in small amounts, can mess up how this ionic liquid works. In supercapacitor prototypes, I’ve seen minor contaminants swing conductivity and electrochemical window. That’s real money and real time down the drain, with results impossible to reproduce. For those running organic syntheses with tight yields, or tuning ionic conductivity in next-generation devices, quality demands close attention to percent purity.
Why Impurities Create Problems
Take water, a classic culprit. Slight moisture in this ionic liquid shows up as shifts in melting point, saps electrochemical stability, and hurts battery cycling. In academic groups, cleaning up for ‘aqueous-free’ purity means days in vacuum or glovebox, then constant checks with Karl Fischer titration. Even trace chloride or other anions—remnants from raw materials or synthesis—impact viscosity, reactivity, and shelf life.
Collaborators in industry echo the headaches. Unwanted ions corrode equipment and complicate waste streams. For those in environmental monitoring or pharmaceutical analysis, impurities lead to false signals, lost resolution, or regulatory headaches. Purity turns from a technical hurdle to a compliance issue before you know it.
Finding the Right Level
Choosing the correct grade means matching purity to your project. Electrochemistry work and battery applications push for better than 99%. Standard grades, in the 98%–99% bracket, suit many synthesis roles or solvent tasks when tiny inconsistencies won’t wreck results. Lower-grade materials might save money for bulk processes, but they will likely introduce risk down the road.
Documentation deserves more scrutiny than most buyers give it. Good suppliers offer complete COAs: NMR spectra highlighting trace peaks, ion content broken down to ppm, and water measured well below 500 ppm for high-grade stock. If a vendor avoids specifics, beware—wishful thinking doesn’t pay off. Research environments—academic, industrial, and regulatory—run better with transparent data.
Realistic Solutions and Supply Chain Strategies
Outsourcing purification only goes so far. Many labs set up drying stations, invest in molecular sieves, or run vacuum distillations as insurance policies, even after buying “high purity.” Sharing sourcing intelligence—comparing COAs, exchanging feedback—helps everyone avoid bad batches.
Building a feedback loop matters most. Find partners who test each lot, adapt cleaning steps, and audit suppliers’ methods. Offer to report back on failed batches; vendors willing to listen and fix problems stand out. Community-driven reviews now fill gaps vendor marketing leaves behind.
Getting the Big Picture
In twenty years of handling tricky chemicals, the story always circles back to purity. Not because it’s a badge of honor, but because it saves labor, sharpens data, and staves off headaches later. For 2-Hydroxy-N,N,N-Trimethylethanaminium Bis((Trifluoromethyl)Sulfonyl)Imide, reliable purity means stronger science and smoother tech transfer. If you want your work to stand up, don’t make excuses—ask for proof, and keep your standards high.
Behind the Shelves: What Choices Say About a Brand
Walking down the grocery store aisle, it doesn’t take long to see how much packaging shapes decision-making. Skim through shelves of coffee or cereal, and the variety jumps out. Some people want enough to last a week, others just want a single serving. Brands know this. They put out family-size, travel packs, and samples. It might sound simple, but these choices reflect how much companies pay attention to regular folks’ lives.
Buying habits shift with work schedules and household size. Parents often buy in bulk to stretch dollars and save on trips to the store. Singles—college students, commuters—lean toward smaller packaging, even if it costs a bit more per ounce. There’s less waste and no worry about food going stale before you use it up. This flexibility makes everyday life smoother, so shoppers don’t have to force a routine around awkward package sizes.
Health, Freshness, and Planet Impact
It’s easy to overlook, but packaging ties directly into health. Imagine sharing an extra-large bag of chips at a party, compared to grabbing a single-portion bag for lunch. When companies offer the right options, they give us tools for portion control. That helps people eat better without feeling trapped by choice—or lack of it.
Freshness plays a similar role; a resealable pouch brings down food waste. Instead of tossing out a half-finished product, it helps keep things tasty longer. Reducing waste helps with grocery budgets and also lightens environmental impact. Less spoiled food equals fewer trips to the trash, which matters more than most realize.
Accessibility and Affordability
Not everyone can spend a big chunk of money at once. When companies stock a shelf with multiple package sizes, they open doors to more customers. A low-cost trial size lets skeptical buyers test something out before committing. Larger packs make sense for those who depend on the same products every week or month. That sort of access lies at the core of everyday fairness.
The details matter in communities where income varies. Offering a spectrum of choices bridges the gap for families on tight budgets and shoppers with unpredictable paychecks. This principle goes back to my own college days. Stretching money meant hunting for whatever fit my wallet, not just my taste buds. Having a cheaper, smaller option made a difference—not just for me but for classmates juggling rent, books, and groceries.
Solutions: What Can Be Done Better?
Practical fixes begin with listening. Surveys, social media, and sales data show what people really want in their cart. Companies can ramp up clear labeling so shoppers see how much product they’re getting for the price. Using recyclable or biodegradable materials in different sizes can chip away at the plastic headache every city faces.
Retailers can work with local groups to see which options really get used. Some places need jumbo deals, others only move snack sizes. Instead of guessing, brands should invest in honest conversation and track what ends up unsold. The best solutions start on the ground, shaped by the people who pick up those packages day after day.