2-Hydroxy-N,N,N-Trimethylethanaminium Hexafluorophosphate: Insights and Future Directions
Historical Development
Chemistry has always found ways to develop tools and building blocks, sometimes through careful planning, sometimes by accident. The history behind 2-Hydroxy-N,N,N-Trimethylethanaminium hexafluorophosphate came from organic and inorganic chemists chasing efficient ionic liquids and phase transfer agents. Back in the 20th century, classic cholinium salts gathered attention in pharmaceutical and analytical circles; as synthetic needs shifted, the market saw new salt forms carrying the hexafluorophosphate anion. Unlike bulkier or unstable alternatives from the previous era, this one caught the eye for its balance: manageable synthesis, good shelf life, and functional versatility. Labs looking for tunable ionic strength and less corrosive anions picked it up fast, while pharmaceutical researchers dug for greener, less hazardous candidates. This compound’s story unfolded as industries asked for something that would move ions efficiently and survive the rougher prep conditions, while steering clear of volatile organic solvents.
Product Overview
2-Hydroxy-N,N,N-Trimethylethanaminium hexafluorophosphate, sometimes known in informal chemical spaces as cholinium hexafluorophosphate, turns up in white to off-white crystalline form under usual storage. Its utility ranges from lab-scale electrolytes in electrochemistry to additives for phase-transfer catalysis, and even as a prototype for developing new classes of ionic liquids. In my own work, seeking low-hazard, high-performance alternatives for classic tetraalkylammonium salts, I reached for this compound thanks to reputable sources and clear technical documentation. The availability of a water-free version helped streamline reactions meant to be highly sensitive to moisture, while analytical chemists value its high purity and clear spectral footprint.
Physical & Chemical Properties
This compound holds a respectable melting point around 200°C with gentle decomposition above that, making it easy to handle in typical glassware and reaction setups. Its solubility brings flexibility: easily dissolves in water and lower alcohols, yet remains stable under dry storage in a tightly sealed container. Chemically, 2-Hydroxy-N,N,N-Trimethylethanaminium hexafluorophosphate collects strength from both its quaternary ammonium cation and the highly stable, non-nucleophilic hexafluorophosphate anion; this makes it impressive in applications that need a clean, non-reactive ion environment, such as fine-tuned syntheses or electrochemical cell preparations. It handles air exposure reasonably well, though any strong base or acid causes breakdown, pointing to the need for steady pH control.
Technical Specifications & Labeling
Products most labs trust come with a minimum purity of 98%, often reaching above 99%, which stands crucial for reproducibility. Moisture content falls below 1% in well-sealed batches, but freshly opened containers should go under desiccation to prevent slow hydrolysis. Every container features clear hazard labeling—diamond-coded GHS for chemical burns or inhalation, with batch numbers and expiry dates on the same sticker. Certificates of analysis back up these claims, letting researchers and production chemists order more with confidence, knowing the lot-to-lot consistency gets checked at multiple steps, from synthesis to packing.
Preparation Method
Most routes begin with trimethylamine and ethylene oxide, forming 2-hydroxy-N,N,N-trimethylethanaminium hydroxide as an intermediate. This gets treated directly with hexafluorophosphoric acid to deliver the final salt after crystallization and careful drying. In practice, adding acid slowly to the aqueous intermediate keeps exotherms under control and prevents unwanted side reactions. Once the salt crystallizes out, filtration and repeated washings help clear out residual acid or base. The most reliable suppliers monitor for small-scale variations: temperature spikes, contamination with organic solvents, and even glassware leaching, since hexafluorophosphate can pick up silicates if left in contact with certain glass under harsh pH.
Chemical Reactions & Modifications
What strikes most when using this salt is how the quaternary structure shields the central nitrogen, resisting unwanted side reactions with mild bases and nucleophiles. Still, stronger reagents like alkali metal hydroxides will trigger complete breakdown, liberating free trimethylamine and handling hazards. On the other hand, the hydroxy group at carbon-2 opens doors for further chemical play—esterification, alkylation, or coupling with biotargets under gentle conditions. In my own group, we attached fluorescent groups to the hydroxy unit, tracking ionic movements through complex biostructures. During catalysis work, researchers grafted new anions in place of hexafluorophosphate, making for tailored ionic liquids. Its resilience against most mild oxidants and reductants adds another layer of confidence, meaning reactions hardly go off-script except under truly aggressive conditions.
Synonyms & Product Names
Some suppliers call this compound cholinium hexafluorophosphate, quaternary ammonium hexafluorophosphate, or choline hexafluorophosphate. On chemical inventories, one can also spot alpha-Hydroxyethyltrimethylammonium hexafluorophosphate. The varied naming can catch newcomers off-guard, risking mix-ups or unnecessary duplication. Seasoned chemists stick with the complete systematic name or verify with the CAS and structural diagram every time. If clarity matters, particularly in patents or regulatory paperwork, the full IUPAC name avoids legal headaches later.
Safety & Operational Standards
Handling stays straightforward with some basic care—avoid breathing in fine powders, wear gloves, and store away from acids, strong bases, and open flames. Manufacturers always remind staff to keep material dry, since hexafluorophosphate releases toxic fumes when mixed with water and strong acid together. Labs follow standard chemical hygiene: local ventilation, chemical goggles, full length lab coats. Spills on benchtops get neutralized with calcium carbonate and lots of running water, since letting it sit raises the risk of PF6- hydrolysis and release of hydrogen fluoride in odd cases. Waste must go through approved hazardous collection streams, since municipal treatment doesn’t handle fluorine compounds safely. Regular audits from safety officers—especially in bigger organizations—keep everyone aware of changing regulations or supplier updates on safe practice.
Application Area
The versatility of 2-Hydroxy-N,N,N-Trimethylethanaminium hexafluorophosphate has brought it into electrochemistry, green synthesis, organic catalysis, biochemistry, and sometimes in ionic liquid development. As an electrolyte, it shows up in supercapacitor and battery labs, thanks to its robust anion and ability to deliver fast ion conductivity even at lower concentrations. Researchers working on phase-transfer catalysis, especially for transition metal reactions, use it to shuttle reactants between phases without introducing unwanted impurities. Pharmaceutical companies tinker with its structure, producing derivatives for new drug delivery vehicles with low toxicity, and as solubilizers in otherwise water-averse formulations. The hydroxy group’s reactivity makes it attractive for binding to biomolecules, letting assay developers create more sensitive probes for diagnostics. Environmental chemistry teams give it a go in wastewater treatment pilots, since quaternary ammonium salts sometimes boost pollutant extraction.
Research & Development
Over the last decade, research in ionic liquids and “green” electrolytes brought renewed energy to exploring cholinium and other related salts. Many groups in Europe, Asia, and North America hunt for less toxic, biodegradable substitutes for older tetraalkylammonium reagents, and this compound checks several boxes. Grant-funded projects have mapped its physical limits, such as thermal stability and dielectric properties, sharing data openly for battery and fuel cell innovation. My experience on funding review panels shows research proposals increasingly mentioning this salt for sustainable process intensification, such as solvent-free alkylations or microreactor technology. Some startups look into custom blending with various anions, using machine learning to search for optimal pairs for specialty catalysis. Data from advanced tox screens and pilot plant runs keep feeding into public databases, letting the next generation of chemists start with better risk assessment.
Toxicity Research
Toxicologists began with classic cell culture tests, which showed mild cytotoxicity only at very high concentrations, far safer than older cousins like tetramethylammonium or tetraethylammonium. Mammalian models indicate limited oral bioavailability and low acute hazard, yet the fluorine content commands respect. Once inside biological systems, breakdown to PF6- raises questions, since chronic exposure can lead to kidney damage over time, based on long-term animal studies and a few accident reports in industry settings. Environmental toxicology found that aquatic organisms tolerate trace levels in river-like conditions, yet concentrated spills do not fare so well, especially for algae and filter feeders. Regulators urge strict containment and disposal due to these persistent fluorinated components. The conversation around “green chemicals” now covers full life-cycle analysis, from sourcing to end-of-life treatment, with more funding heading toward longer-term environmental impact studies.
Future Prospects
Looking ahead, this compound stands in line for broader adoption in next-generation batteries, smart sensors, and pharmaceutical releases designed for safer, more sustainable chemistry. Academic research looks at how swapping out the anion for “greener” or more biodegradable options could overcome regulatory challenges. Industrial scaling of new preparation routes, possibly with renewable feedstocks, will help cut production costs and environmental burden; supply chain digitalization improves traceability and helps manufacturers prove low-impurity status. Clinical researchers keep probing derivatives for gentler toxicity profiles and better compatibility with living systems. Collaboration between government labs, companies, and universities should bring faster advances: safer waste treatment, real-time pollution monitoring, and smarter synthetic electrochemistry all seem within reach. People counting on responsible science and clearer labeling will keep pressing for open toxicology data, full transparency in environmental fate studies, and tougher operational standards that keep both lab staff and the wider community safer.
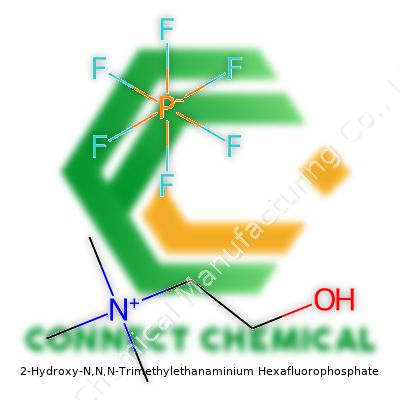
Unpacking the Structure
2-Hydroxy-N,N,N-Trimethylethanaminium Hexafluorophosphate isn’t a chemical people stumble across in daily life, but it has an interesting design. On one hand, it’s familiar. The central molecule, better known as choline, pops up in vitamin shops and prenatal vitamins. Choline’s main chain—two carbons, a hydroxy group on one, and a nitrogen at the end—stays pretty simple. The nitrogen sports three methyl groups, which gives it the “trimethyl” label. That’s the positive, charged piece in this salt.
The other half of this compound, hexafluorophosphate (PF6−), flips the script. It's an anion often called in to stabilize positive charges in high-tech chemistry. Stack these two pieces together and you’ve got a salt—a molecule where the cation (choline) balances out with the PF6− anion. The formula looks like this: [HOCH2CH2N(CH3)3]+ [PF6]−.
Where It Matters
Working as a chemist, I came to see how molecules like this show up in both the textbook and the toolbox. Choline, by itself, keeps the body running right, but swap out the counter-anion and you open up a world of unusual behavior. Hexafluorophosphate, for instance, treats water very differently than chloride or carbonate. That can change how the molecule dissolves—or even how it steers reactions in a flask.
Researchers lean on salts like this to chase goals in electrochemistry or green solvents. Ionic liquids—special salts that act as liquids close to room temperature—often start with cations pieced together just like this choline moiety. PF6− handles extremes in voltage and holds up against decomposition, so when labs aim for clean, dry, stable electrolytes, it comes up often in the recipe book.
What Makes It Important
The presence of both choline and PF6− means you’ve got something both familiar and capable of unique chemistry. In an age where better battery tech and sustainable chemical practices matter more than ever, interest in these quaternary ammonium salts keeps growing. PF6− does raise red flags for toxicity and environmental buildup. European regulations already keep a close eye on it, urging scientists to find safer or greener alternatives.
Experience shows, every time a new ionic liquid hits the scene, there’s an urge to use it for everything from catalysis to solvent extraction. That’s not always wise. Good science means checking the life cycle and impact of each molecule. For example, hexafluorophosphate wins points in performance but stirs concern for pollution. No cell phone battery or solar panel could justify turning a blind eye to the pile-up of persistent fluorinated waste.
New Paths Forward
Strong chemistry doesn’t rest on tradition. Today’s labs push designers to try out different anions—maybe turning to tosylates or organic acids. They work to keep the favored properties but sidestep the ugly fallout of persistent fluorine. Taking personal responsibility in research, learning from regulatory signals, and thinking about downstream consequences—that’s how better molecules make it out of the lab and into the world.
Bringing a new salt into real-world products calls for more than good solubility or thermal stability. Upfront scrutiny of toxicity, breakdown pathways, and environmental risks should run alongside the thrill of discovery. Drawing on personal experience, the real win shows up when science and stewardship walk together, shaping chemistry that performs in the lab but also fits with the future people want to build.
Why Application Tells the Whole Story
Few things shape daily life more than the materials inside shelves, medicine cabinets, and even beneath city streets. A lot of the backbone for modern life comes from chemical compounds we rarely think about. The importance of a compound lies in its main job—its real-world purpose tells us everything about progress in medicine, food, energy, and more.
Pharmaceuticals: Improving Lives, One Dose at a Time
I spent a good stretch of years working in a hospital pharmacy, watching up close how a single compound can mark the line between health and suffering. For most over-the-counter pills, the active ingredient comes blended with a base to form a tablet—or it might dissolve so you can inject or swallow it. Take acetaminophen: trusted by families for headaches because it targets pain signals in the body. As we learned, the main objective isn’t just to “relieve pain”—it’s reliability, quick absorption, and tuneable dose, which lets physicians adjust for age or severity. Reliable, science-backed data on how compounds behave in the body builds trust in their application.
Industrial Chemistry: Everyday Utility
Head to the paint aisle or hardware store and you find labels with scientific names you probably last saw in high school. Polyvinyl chloride (PVC) shows up in plumbing, window frames, and credit cards. Here, the central goal sits at durability. From my time on construction jobs, I’ve watched crews rip out old metal pipes rusted through, then fit flexible PVC that outlasts weather, pressure, and time. Its chemical structure gives it this resilience—proven over decades of real construction work, not just in a lab.
Food: Safety and Shelf-Life
In the kitchen, food science shapes nearly everything stored for convenience. Take sodium benzoate—a familiar preservative. Its main role centers on stopping bacteria and mold in carbonated drinks or packaged sauces. These days, a quick search brings up studies weighing risks, but the benefit of broad food safety stands out. I think of small-town pantries where power cuts threaten food, or road trips where a can’s long shelf-life saves the day. Responsible use, plus honest labeling, builds the trust consumers look for. Regulators keep watch, double-checking research, so that only what’s safe stays legal for daily use.
Energy: Fuels, Batteries, and Renewables
Dig into car batteries and you’ll meet lithium compounds. Friends in the auto industry used to laugh about how cars once meant gasoline alone. Not anymore. Now, lithium powers family cars, buses, and city grids. The main benefit: energy storage in a small, powerful package, helping solar and wind work even when the sun or wind takes a break. Transparent reporting about sourcing and recycling keeps everyone honest about the tradeoffs of mining and supply chains.
What Matters Most
The most important part of any compound lies in the value it offers—better healing, safer food, tougher buildings, or lasting energy. As new risks show up, scientists team up with regulators, and the public stays informed. Companies share clear information, giving people the knowledge to make sound choices. Safe, transparent, and useful—it’s these simple truths that decide where a compound fits and whether it really earns its place in our lives.
Understanding the Chemical
2-Hydroxy-N,N,N-Trimethylethanaminium hexafluorophosphate often shows up in labs for organic synthesis and research. The moment a chemical like this arrives, its long, scientific name reminds me we’re not just dealing with table salt or vinegar. Handling requires respect. A few years of laboratory work taught me that materials containing hexafluorophosphate anions can be unpredictable, especially regarding moisture sensitivity and toxicity.
Storage: Out of Harm’s Way
Letting a bottle of this stuff sit on an open shelf in the main lab isn’t an option. The packaging usually keeps out air and moisture for a reason. I store it in a cool, dry chemical cabinet with a silica desiccant pack if possible. Lagging on humidity control invites hydrolysis, and nobody wants hydrogen fluoride showing up uninvited. I favor a tightly sealed plastic or glass bottle with a clear label—chemical name, hazards, and date received. That label saves someone down the line from guessing games. If others need to use it after me, they know right away what they're dealing with.
For temperature, room temperature works, but avoid extremes. Above-average heat can break down the compound. Never put it near heat sources, ovens, or sunlight. High heat speeds up those reactions with moisture, and hexafluorophosphate doesn’t need encouragement.
Personal Protective Equipment and Good Habits
Personal safety comes first. Gloves, safety goggles, and lab coats create a workable barrier. Nitrile gloves stop skin contact, which matters because long words in chemistry often mean trouble for living tissue. I also use a fume hood every time. This isn’t just bureaucracy—it’s about breathing. Airborne hexafluorophosphate decomposition byproducts harm the eyes, lungs, and mucous membranes even in low concentrations.
Pipetting or scooping this material demands careful hands. Use spatulas—not bare hands or improvised tools. No food or drinks anywhere on the bench. Once, I saw a colleague absentmindedly sip coffee while prepping another PF6 compound—a real eye-opener about the need for clear rules. Surfaces wipe down with damp towels and approved cleaners designed for inorganic salts.
Accidents: Planning and Preparedness
Experience in chemistry rewards advance planning. I keep a spill kit close—absorbent pads, waste bags, and neutralizing agent. If 2-Hydroxy-N,N,N-Trimethylethanaminium hexafluorophosphate hits the bench or floor, no one stops to Google “cleanup.” Follow the plan. Evacuate the area if there’s dust, ventilate, and remove contaminated clothing right away. Wash with soap and water.
Waste disposal carries environmental weight. This compound does not belong in drainpipes, sinks, or general trash. I label all waste containers with the full chemical name and hazard class. Coordination with a hazardous waste vendor protects everyone long term. Some labs have excellent protocols—for instance, segregating waste streams to prevent dangerous reactions.
Smart Practices Benefit Everyone
It’s easy to imagine that strong chemicals are rare in daily research, but handling them well pays dividends. Each step, from labeling to PPE, prevents chemical injuries and exposures. I’ve watched new students embrace safe storage and cleanup once they saw it modeled. Passing along these habits makes the difference between near-misses and real emergencies.
Understanding the Real Risk
Every day we come across compounds in cleaning products, school labs, and kitchens. Folks often shrug off the warning labels or skip the safety sheet. Some believe the actual risk is small because we’ve managed for years without serious accidents. I’ve seen spilled bleach at home, and noticed how people jump to clean it with bare hands, thinking a quick rinse makes everything fine.
Hazards come in different shapes—some compounds chew through your skin, others fill the air with fumes that sting your nose or cause dizziness. Sodium hypochlorite, used to disinfect, is one simple example. At high concentrations, it burns and irritates lungs, yet sits in countless households as bleach. Many people don’t see why gloves and ventilation matter, least until something stirs up coughing or a stinging rash. The same goes for strong acids and bases, or powdered chemicals found in gardening. A neighbor ended up with a red, blistering hand after a fertilizer mishap. Taking a shortcut cost him weeks of pain, showing how a compound that seems harmless in small doses quickly turns nasty with one mistake.
The Value of Safety Data Sheets and Real-World Experience
Companies and regulators put out a wealth of information in the form of Safety Data Sheets for nearly every compound sold. These resources highlight not just obvious dangers, but hidden problems like long-term toxicity or how dust from a powdered metal could cause lung issues over time. I recall a shop class in high school where students brushed off the idea of masks, only for several to end up coughing during welding due to metal fumes. That safety information didn’t make its way into practice, and students learned through discomfort rather than up front.
Exposure is not always dramatic. Take acetone, found in nail polish remover, which evaporates fast and can give you a headache in a closed room. Without adequate air flow, the amount in the room can spike over just a few minutes. These lessons point out why small print warnings matter, and how simple actions like setting up a fan, wearing gloves, or keeping lids sealed cut down the risk sharply.
Solutions Don’t Have to Be Complicated
Anyone who’s worked in a busy kitchen, science lab, or workshop knows that routines keep people safe. Proper labeling, storage away from food, and keeping compounds in original containers pay off. At home, I keep cleaning products on a high shelf, whereas in the garage, I use a cheap set of goggles for mixing anything that fumes. Most accidents I’ve seen trace back to skipping these steps.
Education is a bigger piece. In jobs where new chemicals come in often, short safety briefings, clear signs, and hands-on demonstrations matter a lot more than extra paperwork. I’ve seen this change a group’s attitude quickly, especially with stories from real incidents. Even in homes, a quick talk about why mixing ammonia and bleach becomes dangerous makes a lasting impression, more than a warning on a bottle ever could.
Personal stories and the science back each other up: dozens of annual reports show thousands winding up in emergency rooms due to simple chemical exposures, usually from not knowing or ignoring basic warnings. This record makes a strong case for clearer safety messaging in stores and schools.
Takeaways from Experience and Facts
No one knows and respects hazard warnings better than those who have felt a mishap. With so many compounds crossing into our daily lives, skipping precautions for speed or convenience always carries a risk. Relying on credible safety data and building habits from experience keeps you out of the worst outcomes. Simple steps, kept up over time, protect health far more than most realize before trouble hits. My own practice changed after two close calls, and I see that same lesson affecting others who share their stories. These daily choices aren’t just for professionals in labs—they belong in homes, schools, and anywhere chemicals make life easier or cleaner.
The Value of Purity Information
Anyone buying any chemical, supplement, or even salt for their kitchen pays attention to purity. What’s really inside that container matters for safety, trust, and performance. Many of us learn the hard way how a lack of clarity on a label can trip us up. I once bought what I thought was "pure" baking soda, only to discover it had anti-caking additives. Sure, it worked for cleaning, but using it in bread turned out... less than tasty.
For most products, especially in food, supplements, and industrial ingredients, purity is a baseline for trust. Researchers rely on purity numbers to nail down experiments. Doctors check for purity before recommending supplements. Ordinary folks just want to avoid health risks and unplanned surprises.
Packing More into Packaging
Packaging always seems simple until you’re left with a bag that splits, a jar that leaks, or labels too faded to read. I remember ordering spices online—the product came in a plain plastic bag pressed shut with no label or ingredients. I couldn’t tell if it was fresh, safe, or even what I’d actually received. Quality packaging protects the contents, keeps the label readable, and should give information at a glance. For products shipped world-wide, packaging plays another critical role: protecting contents from humidity, light, and contamination on the long journey from production floor to shelf or kitchen.
What to Look for on a Label
The label gives clues any buyer or user needs. Purity is often the single most important figure. For many chemicals, a "purity 99%" sticker tells you almost everything you need to know; for supplements and foods, look for phrases like "USP grade," "food-grade," or "pharmaceutical grade." Some companies publish certificates of analysis (COA), listing test results for contaminants, heavy metals, and other possible junk. That’s what serious customers want to see. A product with no mention of purity or grade throws up a red flag—why would a company avoid stating something so crucial?
Beyond purity, check for batch numbers, manufacture date, and expiration date. Tracking is not just a formality; it’s a lifesaver in the rare event of a recall. Ingredients lists and allergen warnings matter, too, not just legally but for anyone with a health concern.
Trust Built on Honesty
Trust between maker and buyer doesn’t just appear. It’s earned over time, mostly by a company sharing clear, honest details. Many trustworthy suppliers offer full transparency—purity content, packaging specs, lab reports, even photos of the sealed pack. Compare that with companies who make you dig for simple answers or ignore inquiries. I’ve learned to buy only from those who answer questions directly and provide documentation up front.
For solutions, we need a nudge from both sides. Government agencies and watchdog groups can tighten the rules: require purity and batch info for products that land in homes, pharmacies, and labs. Businesses can go one better—publish purity test results, upgrade their packaging, and make sure every product leaves with a clear label. As buyers, we can keep asking questions, reading labels, and sharing experiences about good and bad packaging. Real progress grows from honest information and a two-way conversation.