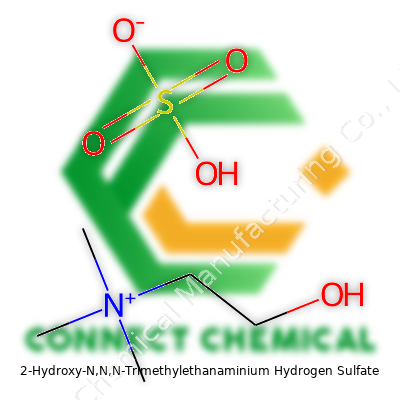
2-Hydroxy-N,N,N-Trimethylethanaminium Hydrogen Sulfate: From Origins to Outlook
Historical Development
2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate holds a quieter place in the annals of chemical engineering—never the showpiece, it often supports other stories. Its roots trace back to pivotal moments in surfactant and solvent design. Chemists started exploring quaternary ammonium compounds seriously in the late 19th and early 20th centuries. Jumping from basic amines to something like this molecule often followed the needs of textile, pharmaceutical, and cleaning industries looking for stability, solubility, and gentler profiles. Out of demand for new ionic liquids, research into this compound’s variants gained speed late last century, mostly in European and East Asian labs building green chemistry platforms.
Product Overview
You’ll find 2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate listed in technical catalogs, usually for roles beyond headline ingredients. It stands as both a phase transfer catalyst and an ionic liquid; its reliability springs from a well-balanced ionic structure, which means it dissolves easily in water and boosts reactivity in organic synthesis. Because it handles both hydrophilic and moderately lipophilic environments, scientists pull it off the shelf for everything from cellulose processing to pharma intermediates. Its comfort in a range of solvents means it shows up in research seeking cleaner, non-volatile paths for classic reactions.
Physical & Chemical Properties
The compound appears as a white and sometimes slightly off-white crystalline solid—sometimes matted, sometimes powdery—absorbing water from the air fairly quickly. At room temperature, it often feels slippery between gloved fingers, hinting at its hydrophilic character. A melting point sitting solidly around 180–190°C puts it among stable salts, and it dissolves in water with little coaxing. Chemically, it doesn’t flinch in neutral or mildly acidic conditions. Its cation holds up to moderate heat and light, while the sulfate counterion provides predictable mineral acid behavior, avoiding unwanted surprises in mixed-salt formulations.
Technical Specifications & Labeling
In most supplier catalogs, purity levels typically top 98%. Buyers keep an eye out for trace metal limits, sulfate residue content, and water percentage. The format—crystals or powder—usually aligns with scale and application, from grams for research to kilos for short-run manufacturing. Proper labeling makes a secure chain: adequate CAS number allocation, batch code tracking, and hazard pictograms ensure accountability from shipper to end user. Good labeling, in my experience, can save hours in audit trails, help manage compliance risk, and, frankly, avoid simple mistakes in busy workplaces.
Preparation Method
Laboratory prep relies on reacting choline (2-hydroxy-N,N,N-trimethylethanaminium) with sulfuric acid—one part organic base, one part mineral acid, water as the medium. The reaction gives off heat, so careful addition matters; too fast and things splash, too slow and runs drag on. After mixing at controlled temperatures, the resulting salt crystallizes out as the solution cools. Filtration and drying under reduced pressure finish the job. In industrial settings, closed reactors and automated dosing systems improve consistency over big runs, but the heart of the process stays true to bench chemistry. Watching a batch crystallize never really loses its appeal, either.
Chemical Reactions & Modifications
This molecule’s most interesting chemistry comes from its quaternary ammonium center and hydroxyl group. Its cation can swap out anions through metathesis with other strong acids or salts, allowing for a laboratory to “customize” the product for downstream applications. The oxidizable hydroxyethyl arm introduces an entryway for functionalization: esterification, oxidation, or etherification happen with the right reagents and conditions. In the field, I’ve used it as a catalyst in alkylation reactions—its basic profile lends speed without pulling the reaction toward unwanted byproducts. As chemists chase greener synthesis, its modification potential marks it as a platform for future innovations.
Synonyms & Product Names
Naming conventions in this corner of chemistry drift between “choline hydrogen sulfate,” “choline sulfate mono salt,” and various formal IUPAC versions. Commercial grades might sport brand-specific names that reflect intended market—pharma, cosmetic, or technical. Some suppliers highlight the “ionic liquid” aspect, especially when targeting clean tech applications. For researchers, sticking to established synonyms smooths collaboration. Avoiding mix-ups matters—one project collaborator received the chloride salt by mistake because of a simple label misread, setting us back a full week.
Safety & Operational Standards
Handling this compound safely involves respect for its sulfate content. Though not outright toxic in moderate doses, exposure can irritate skin and respiratory passages. Labs relying on good ventilation, gloves, and eye protection stay out of trouble. In production settings, spills get absorbed with inert powders and disposed as per local hazardous waste rules. Safety data sheets draw on GHS standards, highlighting best practices for fire, environmental, and chronic exposure. Several guidelines spring from EU REACH compliance. Years in the lab have taught me that respecting these small, “routine” compounds keeps everyone healthy and processes on track—a lesson sometimes reinforced by avoidable accidents.
Application Area
What amazes me about 2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate is its adaptability. In cellulose and biomass processing, it swells stubborn fibers, making them easier to break down—renewable fuel projects greet it as a friend. In pharmaceuticals, it serves as a phase transfer agent, smoothing complex syntheses under aqueous conditions. Contractors in the fine chemical industry lean on its low volatility, using it as a workhorse in catalytic conversions where traditional bases would risk environmental release. Labs working on enzyme stabilization have found that certain choline-based salts support activity in non-aqueous systems, raising efficiency for biocatalysis. In my consulting work, seeing a “niche” compound earn new stripes in sustainable manufacturing always brings optimism.
Research & Development
Labs worldwide keep pushing the envelope on ionic liquid use, with this compound at the core of many experiments. Academics develop greener solvent systems, attempting to tackle long-standing issues in extractions, separations, and catalysis. Its benign profile appeals to teams focused on biocompatibility, especially in drug delivery and diagnostics. Ongoing collaborations between universities and startups promise new insights, combining traditional synthesis with machine learning predictions. I’ve seen firsthand how a simple ingredient can transform a failing process into a scalable solution, not just through bench innovation, but from refining workflow and troubleshooting equipment challenges.
Toxicity Research
Compared to older quaternary ammonium compounds, choline-based derivatives carry a substantially improved safety record. Researchers look at acute oral and dermal toxicity, with studies showing relatively mild effects at practical concentrations. Environmental fate gets plenty of attention—concerns about aquatic toxicity or persistence drive careful assessment. Regulatory agencies ask for full life cycle analysis and extended trials in living systems. My own efforts in compliance often mean tracking evolving toxicity data, sharing results with peers, and adjusting protocols to match the latest requirements. Effective risk communication keeps stakeholders—from procurement to warehouse—on the same page.
Future Prospects
The road ahead looks busy for 2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate. As chemical industries turn toward sustainable models, demand rises for safer, more versatile ionic liquids. Ongoing studies into new synthesis strategies may cut costs and expand access, helping small labs and startups. Application areas could expand, especially as bio-based feedstocks replace traditional petrochemical ones—opening new doors in textiles, surfactants, and agrochemicals. If regulatory frameworks keep pace with innovation, and producers commit to transparent safety practices, this molecule could move from specialty shelves into everyday processes, supporting the next wave of green chemistry solutions. From my own perspective, its blend of dependability and adaptability promises an important role in shaping responsible science and industry.
Understanding the Compound
2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate sounds like a mouthful. Most people might recognize a part of that name—choline. Choline is an essential nutrient and pops up in medical circles, food supplements, and even animal feed. The version with hydrogen sulfate as its counterion isn't exactly a household product, but it serves practical roles where chemistry meets real life.
Roles in Chemistry and Industry
Anyone who’s worked in a chemistry lab or with the pharmaceutical, biotechnology, or food industry will appreciate how raw materials often hide behind long names. This compound stands out because it acts as a source of choline. In water, it dissolves to provide stable choline ions, handy for experiments or production processes that need precise components.
In the synthesis of chemicals, pharmaceutical researchers use it for its clean breakdown and reliable choline supply, especially when clarity about the counterion matters for reaction pathways. In my own experience, clean reactants can shave days off timelines by reducing troubleshooting. Choline comes up as either a supplement or a building block for other molecules. For example, several industrial catalysts depend on quaternary ammonium salts just like this one.
Applications in Animal Nutrition
Feed manufacturers look for efficiency and safety. Most poultry and livestock diets need choline but the traditional chloride-based version sometimes brings issues like corrosion to equipment or unwanted byproducts. Hydrogen sulfate gives a different option, one that cooperates well with bulk blending and doesn’t corrode containers as quickly. You can taste the difference in the air—there’s less of that eye-watering harshness when mixing.
Use in Pharmaceuticals and Research
In pharmaceutical development, choline forms part of treatments for fatty liver disease and neural tube defects, and features in medications and supplements aiming to support brain health. The sulfate-based choline source can improve solubility in some medicinal formulations. This variety may not be as ubiquitous as other forms, but certain experimental processes and sensitive reactions benefit from sulfate’s unique profile.
Considering Safety and Environmental Aspects
People working with chemical compounds must pay attention to their environmental impact. Hydrogen sulfate salts often break down easily in water treatment, so there’s less concern about tricky residue compared with heavier alternatives. In factories, staff appreciate fewer warnings about storing and handling, especially when they don’t have to rush to find a neutralizer for spills. Still, gloves go on, and proper waste procedures remain in force.
Potential Improvements and Broader Access
Practitioners in both research and industry still wish for more consistent supplies. Prices and quality sometimes change, sending folks hunting for reliable sources. Improving manufacturing processes, boosting transparency about ingredient origins, and making safety data sheets easier to read would help everyone—from factory workers to scientists. Wider education about the differences between choline salts can empower buyers to choose the best fit for their needs and reduce costly switching mid-process.
Final Thoughts
2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate sits at the intersection of chemistry and daily work, holding steady as a crucial piece in the puzzle of animal nutrition, laboratory research, and pharmaceutical manufacturing. Real-world results depend on such building blocks being predictable and safe, with room left for more open information and smarter supply chains.
The Substance in Question
Some chemists call it choline hydrogen sulfate. Workers in labs see it as a common ionic liquid. Plenty of folks outside chemistry circles may have never heard the name. This compound is used in research and industry for everything from catalysis to green chemistry projects. The question worth asking: Is it safe to handle, and what steps should be taken to work with it responsibly?
Direct Contact Risks
2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate does not carry the same infamous reputation as corrosive acids, but it's not as gentle as baking flour either. It may cause skin or eye irritation. Many ionic liquids slide under people’s radar because they don’t give off strong fumes, yet irritation risk shouldn’t be ignored. Most people don’t know exactly how their bodies will react after touching lab chemicals for the first or fifth time.
Safety data sheets for this compound flag potential harm from exposure. This can mean redness, a burning sensation, or worse, depending on length of contact and personal sensitivity. Inhaling dust from powders or dried residues carries risks for the nose and lungs. Accidents rarely come with warning labels. In a shared lab setting, assumptions break things. Responsible users always reach for gloves, goggles, and—if possible—a lab coat. I’ve seen chemical safety habits turn minor spills into nothing more than an inconvenience instead of a call to emergency response.
Impact on the Environment
Few laboratory chemicals break down overnight. Many stick around in water, soil, or air, affecting what lives there. Ionic liquids, including this choline salt, were once touted as “green” replacements for some traditional solvents because they don’t evaporate as quickly and produce less atmospheric pollution. That’s only one part of a much larger picture. Persistent organic chemicals can build up quietly. Mistakes in disposal can carry consequences downstream. Most environmental studies on this salt suggest it’s less harmful than old-school solvents, but less harmful does not equal safe to ignore.
Good practice means careful collection and disposal, labels on storage containers that actually stay attached, and treating all spills quickly—before someone walks through a puddle or a drop runs into the sink. I’ve met too many new lab workers who figured water could wash away anything. Nature disagrees.
Improving Safety on the Ground
Companies and educational labs often rely on printed safety posters and mandatory once-a-year training videos. In my experience, real progress comes from mixing hands-on learning with clear communication. Most new researchers gain confidence in chemical handling through mentors, not manuals. Sharing stories about close calls makes more impact than reciting regulations. Repeating steps for proper storage and explaining why goggles save eyesight can turn risky habits into automatic actions.
Clear labeling, tidy workspaces, and reliable access to safety gear help stop problems before they start. Supervisors and experienced workers set the tone by actually following the rules themselves. Cutting corners—either to save time or due to overconfidence—ends up backfiring, sometimes in dramatic fashion. You can’t erase a chemical burn, but you can prevent one.
Solutions Within Reach
Building a responsible culture happens through small, steady habits. Regular refresher courses, peer-to-peer reminders, and leadership that highlight mistakes as learning chances—not failures—create safer lab environments. Chemical manufacturers offering updated and easy-to-read safety data can prevent confusion for workers new to the material. Suppliers who include explicit disposal guidance help reduce guesswork.
Every new chemical, from ionic liquids to the classics, deserves attention and respect. A little caution saves skin, eyes, and peace of mind.
Real Life Meets Science On The Shelf
Many forget how much simple storage determines product quality. Walk into any supermarket, and it’s easy to grab a carton of milk or a box of cereal and not give shelf conditions a second thought. Before my first job in quality assurance, I took for granted that all foods kept their taste and value just by sitting on the shelf. After opening a stale bag of chips once too often, I learned just how wrong that attitude can be.
The importance of temperature, humidity, and light exposure goes beyond just getting the best flavor. I’ve seen vitamins lose their punch after baking the entire summer near a sunlit window. I’ve also watched whole boxes of medicines end up in the trash after a few weeks of too much heat. Evidence supports these experiences. For instance, the U.S. Food and Drug Administration warns that improper temperature can cause drugs to degrade up to 70% faster.
It’s Not Just About Food
Take electronics as another example. Phone batteries stored in humid or hot warehouses risk swelling, leaking, or worse. The same trouble follows paints, adhesives, and even simple cleaning agents. Forgetting to check storage rooms cost a local car repair shop thousands when water seeped into their oil barrels. Every time I visit their cold, tidy storage locker now, I appreciate the lesson they had to learn the hard way.
Why Storage Instructions Deserve Attention
Manufacturers put storage details front and center for reasons built on science. Safe storage prevents mold, bacteria, chemical changes, and even explosions. Just a few degrees outside the safe range can jeopardize everything. Studies repeatedly show that shelf life, potency, and safety depend directly on how the product survives before it ever leaves the warehouse.
People sometimes skip over labels or misunderstand directions. “Keep in a cool, dry place” isn’t just a suggestion—it’s a warning. My grandmother’s habit of putting canned goods next to the stove shortened their lifespan and, once, ruined a batch of tomatoes. Heat creep caused the cans to bulge and pop. Learning from her, I started reading every storage instruction as though safety depended on it, because in some cases, it does.
What Can Actually Help
Getting storage right starts with education and small habits. Shop owners who train their staff to check temperatures and rotate stock rarely run into those “what’s that smell” moments. At home, just keeping products off the floor and away from heat sources does more than most cleaning sprees ever could. Investing in basic thermometers and using proper shelving changes the game for perishable products, electronics, and chemicals.
Simple checks save money and health. Look for faded labels, ask workers about recent inspections, and don’t be shy about reporting odd smells or bulges in packaging. These steps draw on real stories from people who have lost batches, ruined inventory, or faced recalls. They have learned, sometimes at high cost, that the way storage conditions are managed will decide how safe and usable a product remains.
Respect for storage recommendations doesn’t only protect business interests. It guards the health of families and customers. Mistakes in shelving, lighting, or temperature will show up in the quality and safety of any product, whether it ends up in a lunchbox, a medicine cabinet, or a toolbox. Pay attention to those small warnings—they often make the biggest difference.
An Insight into Its Formula and Mass
2-Hydroxy-N,N,N-Trimethylethanaminium hydrogen sulfate carries a name that takes some getting used to. In practical life, it’s more widely recognized as choline hydrogen sulfate. The chemistry world doesn’t hide its rules. You can break the name apart to see what's in the molecule: a choline cation and a hydrogen sulfate anion. Putting these details together gives the chemical formula C5H14NO•HSO4. Written out, that’s C5H14NO+ for choline, then HSO4− for the hydrogen sulfate. They balance each other electrically.
Why Formula and Molecular Weight Matter
In daily work, especially in labs, knowing exactly what you’re holding changes the safety routine, storage plans, waste disposal procedures, and even simple observations, like color or solubility in water. Numbers like molecular weight also come up every time someone tries to figure out how much of the substance fits into a solution or how it behaves once mixed with others.
Speaking of numbers, the molecular weight for choline hydrogen sulfate turns out to be 185.24 g/mol. To get there, just add up the individual atomic masses: five carbons, fourteen hydrogens, one nitrogen, one oxygen for the choline; an extra hydrogen, one sulfur, and four more oxygens for the hydrogen sulfate half. Reliable references and calculators confirm that value.
Importance Beyond the Classroom
Choline-based salts draw interest from biochemists to green chemists. Choline is vital in animal nutrition and acts as a building block inside living cells. On a different track, the sulfate piece often plays a supporting role, adding solubility or helping to buffer acidity. The two together—choline and hydrogen sulfate—produce a salt that dissolves in water, blending easily into broader processes.
What makes this compound notable goes beyond its role in a textbook. It’s common in ionic liquids, known for their use as “green solvents.” In recent years, ionic liquids like choline hydrogen sulfate have been celebrated for their low volatility, lower toxicity, and ability to replace more hazardous traditional chemicals. My time in a laboratory showed me how quickly reactions proceed in their presence. Choline salts let researchers test out reactions that struggled or failed in conventional solvents.
Balancing Progress with Safety
Every introduction of a new chemical creates questions about risk. Choline and sulfate ions both show up naturally in living things, but that doesn’t make every combination safe. Accurate formulas and molecular weights help with hazard labeling, regulatory compliance, and emergency response, especially in industries scaling up production.
Many see solutions in supporting more research with complete transparency—shared laboratory findings, open databases, and honest risk assessments. Chemists and safety officers benefit when data on substances like choline hydrogen sulfate are shared freely. Better safety data sheets, more robust training, and routine review of storage protocols protect workers and the environment.
In my own experience, keeping up with chemical details pays off. Staying responsible with new materials means always checking and double-checking physical and chemical data. We learn more each year about their benefits, limits, and how to handle risks. That sense of vigilance, rooted in solid information like formulas and weight, builds a culture of safer innovation.
Why Some Products Need Careful Disposal
Throwing something away rarely ends with tossing it in a trash bin. Landfills keep growing, and many don’t realize that seemingly ordinary products can pack a punch when buried or burned. Electronics, batteries, and everyday cleaners often hold more than meets the eye. If a phone or laptop lands in a landfill, heavy metals like lead or cadmium can seep into the ground. Household chemicals, paints, or pesticides can spread toxins. Incineration doesn’t always solve the problem—some substances create even nastier fumes. Local water supplies and soil can get tainted quickly, harming plants, animals, and people.
Real-World Consequences From Skipping Proper Disposal
Word travels fast in environmental circles because mistakes show up in health reports. The US Environmental Protection Agency points to growing lead and mercury levels in groundwater, often traced back to improper disposal of items such as thermometers and batteries. Personally, I’ve seen what happens when someone in my old neighborhood dumped an old car battery in a garbage pile behind a house. Within weeks, neighborhood cats started acting sick. Local vets found high lead levels, and soil tests revealed contamination that lingered for years.
Hazardous waste isn’t a far-off problem. In the United States alone, up to 3 billion batteries head to landfills each year. The World Health Organization notes how improper chemical disposal creates health problems in children—including respiratory issues and chronic fatigue. The fact is, some products build up in the body and can’t be removed.
Where Companies and Shoppers Drop the Ball
Companies sometimes assume people know how to deal with their old products, but the truth is, clear labels and instructions rarely show up on packaging. Consumers forget about small print or skip reading, so batteries go in the bin with candy wrappers. Without strong collection programs or clear drop-off points, even folks who want to do the right thing end up confused. States and cities often post recycling signs, but not everyone catches those messages.
Public trust depends on clear, honest communication from manufacturers and government agencies. If companies call attention to special disposal, people can protect themselves and their communities from accidents. When buyers understand what's inside gadgets, lightbulbs, or aerosol cans, safer habits follow. A neighbor once organized a “take-back” day in our community. Folks brought in piles of electronic waste and paints. Kids learned about the ups and downs of recycling, and we all felt we’d kept something bad out of the ground that day.
What Everyone Can Do Differently
Building better habits doesn’t always need new laws. Supermarkets and hardware stores can set up bins for batteries, bulbs, and electronics. Schools can educate kids about basic hazards hiding in their homes. As a parent, I keep a separate box for used batteries and electronics until my town’s quarterly drop-off day. Chatting with the local recycling center helps, too—they’re usually happy to explain what goes where.
Everyone can ask more questions: Is this product safe to toss? Should it go somewhere special? Staying curious, checking local rules, and teaching our kids to do the same slowly adds up. Simple steps from individuals and honest messaging from brands and governments can keep our water, air, and neighborhoods cleaner for everyone.