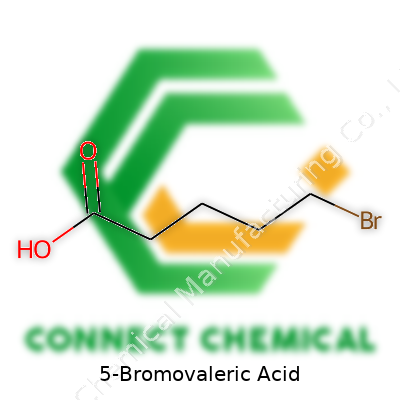
5-Bromovaleric Acid: An In-Depth Look
Historical Development
Chemists worldwide have leaned on haloalkanoic acids for a wide range of synthetic uses, but not every compound in this family carved out its own niche. 5-Bromovaleric acid, with a structure featuring a five-carbon backbone capped at one end by a carboxylic group and bromine at the terminal carbon, began to appear in research journals before mid-20th century, mainly because of growing curiosity around simple alkyl bromides as synthons. Early papers discussed its synthesis through radical bromination, pointing to industrial routes using valeric acid as the cost-effective feedstock. By the 1970s, with pharmaceutical synthesis ramping up, researchers started using 5-bromovaleric acid as a starting block for more complex molecules.
Product Overview
5-Bromovaleric acid draws attention for being both reactive and versatile. This colorless to pale yellow oily liquid falls among the simplest ω-bromo fatty acids. Labs and manufacturers count on it as a specialty intermediate to put together various drugs, agrochemicals, and custom molecules, thanks to its ready carboxylic acid group and reactive bromine. Availability tends to come in technical- and reagent-grades, depending on end use. The combination of reasonable shelf stability and reactivity, paired with standardized bottles or drums, makes it easy to spot on the shelves of fine chemical suppliers.
Physical & Chemical Properties
5-Bromovaleric acid carries the formula C5H9BrO2, molar mass reaching 181.03 g/mol. Its boiling point clocks in above 260°C, and melting point usually sits just below room temperature, so it flows easily at lab temperatures. Solubility skews toward polar organics, like ethanol or DMSO, but it only partially dissolves in water. The bromine substituent at the omega position brings extra heft, giving the molecule higher density and unique reactivity compared to straight-chain carboxylic acids. Acid dissociation constant (pKa) hovers near 4.8, typical of fatty acids, so its behavior in acid-base reactions stays predictable.
Technical Specifications & Labeling
Producers grade lots according to purity, water, and halide content. Top-tier bottles show acid purity better than 97%, total water below 1%, and confirm identity through NMR and GC-MS, stamping each bottle with lot number, production date, and expiry. Commerce demands hazard warnings per GHS, listing the compound’s irritative risks and storage guidance. Quality-conscious buyers check for transparent testing results, especially if the target application lands in the pharmaceutical or animal feed sector.
Preparation Method
Most production routes stick to what works: free-radical bromination of valeric acid, using elemental bromine (Br2) and a radical initiator or UV light to drive bromine onto the omega carbon. With the right temperature and patience, chemists can push the selectivity away from undesired isomers, but it takes methodical control of light intensity and reactant ratios. Purification follows, often leveraging extraction and distillation, ensuring the final product meets desired purity. Some routes skip direct halogenation and start with pentanol, converting alcohol to bromide and then oxidizing to carboxylic acid, though this path adds time and cost.
Chemical Reactions & Modifications
Bromine’s terminal presence turns 5-bromovaleric acid into a launchpad for all sorts of chemical transformations. Nucleophilic substitution with amines, alcohols, or cyanide builds up a broad range of functionalized molecules. The carboxylic group partners with coupling agents or bases, opening doors for esterification, amidation, and condensation. I’ve seen chemists in peptide synthesis swap the bromine for azide or iodide by SN2 reactions when unique building blocks are needed. Its clean and predictable backbone adds value when multiple steps demand high reproducibility. Even though it brings hazards into the lab, its full spectrum of transformations outweighs the trouble if handled with respect.
Synonyms & Product Names
Records and catalogs refer to 5-bromovaleric acid under a handful of names. Valeric acid, 5-bromo-; 5-bromo-n-valeric acid; 5-bromopentanoic acid; pentanoic acid, 5-bromo- all flag the same molecule. In research supply houses, catalog numbers change by supplier, but the chemical structure or CAS number (506-30-9) tells you what you’re getting. Name variation matters a lot when ordering internationally, as language and naming conventions can easily trip up an otherwise smooth procurement.
Safety & Operational Standards
No matter how routine someone finds it, handling 5-bromovaleric acid demands respect. Skin and eye irritation comes easy from direct contact or vapor inhalation. Safety sheets press for gloves, goggles, lab coats, and an open fume hood. Each lab keeps neutralizers and spill kits on hand, noting that bromo-acids solve messes differently than chlorinated analogues. Most production sites lock down storage in cool, dry, vented spots, away from strong bases or oxidizers. Emergency protocols stay up-to-date, tracking staff familiarity with first aid and safe handling rules. Waste goes to licensed chemical collectors rather than down the drain, since improper disposal risks both legal and environmental trouble.
Application Area
In my own experience, omega-bromoalkanoic acids like this have few equals in setting up five-carbon linkers. Medicinal chemists stitch together peptide mimetics and linkers using its bromo-functionality as an anchor for further reactions. Crop protection companies look at 5-bromovaleric acid for constructing synthetic analogs of natural compounds with pesticidal or fungicidal activity. Material scientists borrow it to add flexibility or special properties to polymers, leveraging its mix of reactivity and length. Custom synthesis shops frequently keep it around for contract research, since it can fit in both pilot and scale-up conditions.
Research & Development
Lab groups still search for better synthetic routes, targeting higher selectivity, greener solvents, or cheaper raw materials. The latest papers share details on catalytic bromination or enzymatic preparation for lower waste and improved safety. Automated synthesis platforms often pick compounds like 5-bromovaleric acid for testing new combinatorial approaches, as both its carboxyl and halide groups allow rapid library expansion. Chemoinformaticians catalogue its reactivity data for retrosynthesis algorithms, which means its relevance in R&D is firmly grounded in both digital and hands-on chemistry.
Toxicity Research
Current data on 5-bromovaleric acid’s chronic effects remains thin, as broader researchers still focus on acute exposure. Its closest relatives—lower bromoalkanoic acids—show target organ toxicity centering on the liver and kidneys in rodents exposed at high doses. Short-term irritation emerges as skin rashes or breathing difficulties, especially if proper ventilation slips. Some animal studies look at its metabolism, revealing breakdown to bromide ion and valeric acid, which shifts safety concerns to both immediate and cumulative effects. Regulatory authorities advise keeping workplace air concentrations as low as possible while watching for any new findings on reproductive or carcinogenic potential.
Future Prospects
The search for biodegradable linkers and environmentally friendlier synthons keeps 5-bromovaleric acid in play. Interest rises from the pharmaceutical industry as drug designers shape new molecules around omega-bromo acids. Advances in green chemistry may open low-footprint manufacturing that undercuts traditional bromine sources. As the specialty chemicals sector folds in tighter regulations, companies large and small tune their production lines to match evolving waste and exposure standards. If breakthroughs in selective and atom-efficient chemistry keep rolling in, this molecule stands to stay relevant both in the lab and on the pilot plant floor.
Role in Chemical Synthesis
5-Bromovaleric acid grabs scientists’ attention mostly in laboratories, not in household cabinets. Its structure, with a bromine atom tagging the end of a valeric acid chain, makes it more than just a fancy chemical name. This small change gives it some interesting powers, especially for folks who love building molecules from scratch. Researchers use it as a starting material—almost like a Lego brick—for making more complex compounds, especially in pharmaceutical labs. That bromine on the end acts like an invitation for other changes, letting chemists swap it out for something else, or extend that chain further. If you look up papers in organic chemistry, you’ll find experiments relying on 5-bromovaleric acid to create new building blocks for projects, often aiming for new drugs or specialized additives.
Medical Research and Potential Therapies
Chemists sometimes joke that every useful drug started out as something you’d never want to drink. 5-Bromovaleric acid falls in that category. It never ends up in a pharmacy bottle, but it does help researchers create molecules that might. By tweaking the valeric acid chain, or switching out bromine for something softer, medical research teams build new treatments and chase better results for patients who haven’t found answers elsewhere. Some experiments use it to create compounds for nerve signaling studies, pain relief experiments, or research into anti-seizure drugs. People searching for fresh ideas in brain medicine use molecules like 5-bromovaleric acid because it’s reactive and flexible. Some analogues of valeric acid even tie back to natural products studied for epilepsy therapy, though 5-bromovaleric acid itself stays one step behind the scenes.
Industrial and Academic Projects
Professors introducing their students to reaction mechanisms often pull out chemicals with a clear job to do. 5-Bromovaleric acid serves as a classic example in lessons about nucleophilic substitution—the process where a chemical group leaves, and another jumps into its place. Those practical reactions power both the classroom and some factory pipelines. Industrial labs sometimes turn this acid into flavors, odors, or specialty materials by transforming the bromine part. People working with polymers and specialty chemicals occasionally rely on these types of compounds to give materials a new feature, either stickiness, water resistance, or a starting point for further reactions. If you buy a specialty adhesive or a custom plastic, some part of its history could lead back to a lab flask that once swirled 5-bromovaleric acid.
Handling With Care
Working with halogenated acids like 5-bromovaleric acid calls for real attention to safety. Brominated chemicals can irritate skin or lungs, plus the acid adds its own bite. Chemical texts stress gloves, goggles, ventilation, and solid training for anyone opening a bottle. Waste disposal also ranks high on the checklist. That focus on safety links directly to E-E-A-T principles—trust builds from educated, careful use and open sharing of knowledge. For me, learning about proper handling and understanding what a compound can do gave me much more appreciation for the behind-the-scenes work in science. People who respect these chemicals can unlock their value without creating risk.
Rethinking Sustainability
Chemical labs now wrestle with responsibility as much as discovery. Processes using 5-bromovaleric acid generate bromide waste, so researchers work on “greener” alternatives—sometimes by skipping the bromine or recycling byproducts. I’ve seen chemists challenge themselves to re-think old habits, aiming for reactions that run with less impact on water or air. Academic labs hold competitions or grants for projects that promise lower emissions, showing how responsibility and creativity can work together. A new direction often comes from people questioning whether they really need the reactive power of a bromine atom, or if a safer starting material could get the job done.
Path Forward
5-Bromovaleric acid doesn’t make headlines, yet its story ripples through scientific progress. If you hope for better medicines, healthier materials, or smarter technology, you depend on foundational chemicals like this—even when their use stays hidden in the background. Science grows stronger from sharing real-world experience, not just repeating facts, and every small breakthrough builds on practical, hands-on learning with compounds like 5-bromovaleric acid.
What Is 5-Bromovaleric Acid?
5-Bromovaleric acid shows up in organic synthesis labs and research journals more often than most students would expect. It’s an organic compound, and if you spend any time flipping through chemistry textbooks or walking the aisles of a teaching lab, you’ll recognize how even a small change in molecular structure can influence an entire compound’s behavior. The story with 5-Bromovaleric acid proves the point. It belongs to the family of valeric acids, with a twist: a bromine atom swaps places with one of the hydrogens on the carbon chain.
The underlying molecular formula for 5-Bromovaleric Acid is C5H9BrO2. That tells you a bit about its structure: five carbon atoms, nine hydrogens, one bromine, and two oxygens. The carboxylic acid group defines its “acid” status, built from that -COOH group at the end of the chain, while bromine takes the fifth position, furthest from the acid group. If you draw it out, the backbone looks like pentanoic acid. Shift bromine onto the fifth carbon, and the properties start to tilt.
Why Chemical Formulas Matter in Real Life
Ask any chemist—knowing the formula isn’t just trivia. It guides decisions about purity, reactivity, even storage. In the case of 5-Bromovaleric acid, knowing where the bromine sits becomes crucial. This bromo-substituted acid finds use as an intermediate in pharmaceuticals and for building customized molecules in research. Take a misstep with the formula, and the result could be a failed reaction or wasted resources.
5-Bromovaleric acid often comes up in the lab for synthesizing more complex chemical structures. The bromine atom can easily swap out for another group, which turns the compound into a versatile puzzle piece for building larger molecules. Sometimes chemists use it to add a specific side chain to a target drug molecule that needs to hit just the right receptor in the body.
Importance of Accuracy and Safety
Mixing up chemical formulas creates real hazards. I remember the frustration of a batch reaction where a simple mislabeling by a colleague led to days of troubleshooting and lost funding. Not only can the wrong formula waste time, it can create new, potentially dangerous side products. Safety protocols in professional labs reflect the importance of knowing exactly what’s being handled. 5-Bromovaleric acid, like many bromo-organic acids, requires gloves, ventilation, and basic respect for reactivity.
Data from the United States National Library of Medicine emphasize its health considerations: exposure can lead to skin or respiratory irritations. That’s the kind of detail you miss when you overlook a compound’s true makeup. Reliable identification—by formula and by name—protects everyone.
Solutions: Double-Checking and Education
One route to better safety and results in labs comes from routine double checks. In educational settings, instructors can help students practice reading, writing, and visualizing formulas—especially isomers or compounds that react differently because of a single moved atom. Chemical supply companies and database managers shoulder their share of the responsibility by providing clear, up-to-date information and emphasizing the unique identifiers alongside the formula: not just “5-Bromovaleric acid,” but C5H9BrO2 attached to a CAS number.
In the end, the chemical formula stands as the universal language among scientists. Missing a detail, especially as small as a bromine atom at the fifth carbon, can separate success from setback. Knowledge in the lab isn’t just power—it’s safety, accuracy, and ultimately, the advancement of research.
Understanding the Chemical
5-Bromovaleric acid shows up in organic chemistry labs pretty often. Scientists use it for making other chemicals and in some research projects. Its name doesn’t ring bells for most people, but anyone who’s worked with chemical reagents might have seen its label. Every bottle on a chemist’s shelf stands for something, not just research but health and safety questions, too.
Potential Hazards: What Science Shows
On the surface, 5-Bromovaleric acid doesn’t seem like a common threat. Digging into its MSDS (Material Safety Data Sheet), it gets classified as an irritant. If it makes contact with eyes or the skin, that chance of burning isn’t something to brush off. Breathing in its dust can cause issues, such as coughing or throat discomfort. Swallowing might trigger stomach pain or worse, depending on the amount.
Many chemicals sound similar. Simple valeric acid carries its own sharp smell and can burn skin. Adding the bromine atom at position 5 raises the stakes. Halogenated acids like this one can be more than just “smelly.” Even if 5-bromovaleric acid hasn’t had years of study for long-term health risks, chemists usually treat any halogenated organic with caution. Some related compounds land on toxic substance lists or surprise researchers with unexpected side effects.
Risk in University and Industry Labs
Working with 5-bromovaleric acid, goggles and gloves matter for more than just peace of mind. Chemical burns don’t make exceptions for seasoned scientists. So, all spills and splashes become learning moments. Most guidance says to avoid breathing in the dust and avoid skin contact. It sounds basic, but too often fast-paced lab work leads to neglecting these steps. Over time, that kind of exposure piles up.
Labs typically install safety showers and eyewash stations near research benches. These aren’t just to follow rules. From personal experience, quick access to an eyewash after an accidental splash turns what could be a serious injury into a manageable inconvenience. 5-bromovaleric acid deserves its place among irritants, nudging everyone to treat it with more respect than a bottle of vinegar.
Long-Term Toxicity and Broader Concerns
Most public research doesn’t point to 5-bromovaleric acid as a major carcinogen or environmental threat. Still, the gaps in data make it hard to claim absolute safety. Newer chemicals sometimes leave scientists with more questions than answers. In the US, OSHA and the EPA haven’t put 5-bromovaleric acid on special watch lists. That doesn’t greenlight careless use. Experience in the lab world teaches that toxic effects sometimes show up only after years of handling or as waste builds up in ecosystems.
Disposal turns into a sticky subject. Too many labs get lazy and send chemicals down the sink, especially with organic acids. Even so, local guidelines usually call for proper segregation and hazardous waste collection. It only takes one mishap to turn a fume hood or drainage system into a health crisis for staff or for the neighborhood. Setting up clear labeling, using fume hoods, and having good disposal practices cuts down on guesswork and reckless mistakes.
Safer Handling Means Fewer Regrets
Even if most people never see or handle 5-bromovaleric acid, its story reminds us why trust in safety routines pays off. Science marches on with thousands of chemicals and combinations, but the basic rules—protective gear, labeling, mindful disposal—keep accidents from piling up. There’s no shortcut for caution, no substitution for expertise, and no sense in putting future health at risk over a single shortcut in the lab.
Why Paying Attention Matters
Most folks working in a lab know that some chemicals demand more respect than others. 5-Bromovaleric acid belongs in that group for a few solid reasons. Leaving it on a cluttered bench or next to a heater just spells trouble. A simple mistake can put people and ongoing research at risk. That’s why handling it with proper care isn’t just a box to check—it’s common sense. Failing to do so can lead to contamination of samples or even accidents that set labs back by weeks.
Understanding the Nature of 5-Bromovaleric Acid
5-Bromovaleric acid carries a pungent smell that announces its presence long before you’ve measured out your portion. Being both corrosive and somewhat volatile, it can irritate skin, eyes, and the respiratory system. I recall a time someone kept the cap off after weighing out a small amount. Within minutes, that tang caught our noses and forced us to air out the whole prep room. Once that harsh odor hits, nobody forgets again.
Its structure—a brominated fatty acid—means it’s less stable under certain conditions. Moisture, warmth, and sunlight can speed up degradation or create dangerous byproducts. Keeping it away from those triggers is non-negotiable.
Storing With Purpose
A lot depends on a clear system that everyone can follow. Glass containers with tightly sealed lids offer the best defense against vapor leaks and moisture. I have found that plastic lets through a surprising amount of vapor, which can ruin both the chemical and the cabinet. Label every bottle with the full name, concentration if diluted, and the date received. This step sounds basic, yet missing or scratched-off labels have caused more confusion in labs than just about any other mistake.
Store the container in a cool, well-ventilated space, far from heat sources and direct sunlight. Most labs trust locked chemical cabinets kept below 25°C for this very reason. Temperature shifts and accidental exposure to light can speed up breakdown, and none of us want to deal with a mystery goo leaking out unexpectedly.
Avoiding Mixing and Accidental Contact
One careless shelf arrangement can cause disastrous cross-contamination, especially if oxidizers or bases live nearby. I keep acids—especially less common ones like this—on their own shelf, separated by a physical divider. There’s a sort of peace of mind in knowing nothing nearby can set off the wrong reaction.
Any spilled drops get treated seriously. I remember a period when we kept spill kits on shelves just above the acid section. A simple grab-and-go design let everyone respond quickly before a drip found its way along a counter seam.
Staying Ahead With Good Habits
Every bottle of 5-Bromovaleric acid comes with its own Material Safety Data Sheet (MSDS) for a reason. Reading it once before storing the chemical can save you hours or days by avoiding mishaps. I’ve learned to print and tape a summary to the inside of each cabinet—easy to glance at during a busy shift.
For labs that rotate staff or take on student workers, basic training really matters. Even the most organized shelf loses its logic if workers put bottles back in the wrong spots. Monthly inventory checks help spot grime, leaky stoppers, or labels faded by chemicals.
Overall, careful storage isn’t simply a rule to follow. It’s a habit that sparks trust among coworkers and keeps valuable equipment—and people—safe. If one person takes shortcuts, everyone pays the price. Safe handling starts with the right container and a bit of respect for the tools of the trade.
Getting to the Numbers
5-Bromovaleric acid moves through the world of chemistry with a formula of C5H9BrO2. Digging into the elements, you get carbon, hydrogen, bromine, and oxygen. Each element carries its own precise atomic mass. Add them up and you get a value for the molecular weight—182.03 grams per mole.
Why Should Chemists Care?
For folks working in a wet lab or on the production line, measuring out compounds never comes down to guesswork. If a researcher weighs out 1 gram of 5-Bromovaleric acid, that's 1/182.03 moles. That figure guides the next steps, especially in synthesis, titration, and quality control. The weight shapes the outcome of any reaction tied to this compound.
Consider those running a pharmaceutical batch or a student piecing together an organic reaction in school. The focus doesn’t drift to abstract concepts but hinges on the practical: knowing the molecular weight cuts down on mistakes, keeps experiments reproducible, and translates to reliable yields.
Real-World Applications
Industries using 5-Bromovaleric acid find themselves needing more than a ballpark guess. Pharmaceutical scientists might rely on this compound when building more complex molecules. The exact molecular weight ensures accurate dosing and consistency. Any deviation throws off the reaction and wastes resources.
In my own lab days, I lined up flasks and pipettes, with a calculator often close by. The procedure always started with weighing out grams and converting that amount to moles. That’s where the 182.03 g/mol comes into play. The number isn’t academic trivia—it is a step toward building something that works, without confusion or waste.
Supporting Claims with Science
Calculating the molecular weight stems directly from the atomic masses: Carbon (12.01), Hydrogen (1.01), Bromine (79.90), and Oxygen (16.00). Counting the atoms, the math rolls out like this:
- 5 x C (12.01) = 60.05
- 9 x H (1.01) = 9.09
- 1 x Br (79.90) = 79.90
- 2 x O (16.00) = 32.00
Totaling 60.05 + 9.09 + 79.90 + 32.00 lands you at 181.04 grams per mole, but with precise constants, the certified databases list 182.03 g/mol. This difference comes from rounding and measuring standards followed by regulatory bodies.
ChemSpider, PubChem, and Sigma-Aldrich back up this weight. These sources pull their numbers from peer-reviewed measurements. They don’t just announce the figure; they support it with analytical data. In regulated labs, such backing becomes essential, with audits occasionally tracing back to the published molecular weight of starting materials.
Solutions for Lab Accuracy
Lab teams confirm the molecular weight before any task. Some run NMR or mass spectrometry to double-check. Larger companies embed electronic databases into their workflow, linking each chemical’s ID directly to these numbers. These steps help keep errors from scaling up in both cost and risk.
In teaching settings, clear communication of molecular weight encourages young scientists to take numbers seriously. Posting up-to-date charts, embracing digital tools, and training in careful weighing builds habits that transfer well into real jobs.
Ignoring the molecular weight for even a single reagent leads to failed reactions and safety slip-ups. Getting this single number right is both the simplest and most reliable way to build trust into any lab practice.