5-Bromovaleryl Chloride: A Deep Dive into a Key Fine Chemical
Historical Development
Chemicals like 5-Bromovaleryl chloride have pretty interesting backstories in research and industry. Many halogenated valeryl chlorides first gained momentum in labs during the later parts of the 20th century, especially as researchers dug into new pathways for pharmaceutical synthesis and advanced materials. As folks searched for building blocks to make specialty molecules, brominated intermediates drew attention because the bromine atom brings a unique reactivity. 5-Bromovaleryl chloride isn’t exactly a headline stealer, but its presence shows up in patents, academic publications, and supplier catalogs, marking its steady climb into the toolkit of chemists who want reliable, straightforward chain-elongating agents. Compared to the earlier popularity of more basic acyl chlorides, brominated versions like this one symbolize a shift towards more targeted synthesis and the hunger for functional groups that open doors to unique transformations, particularly in the chase for new drugs and crop protection chemicals.
Product Overview
To put it simply, 5-Bromovaleryl chloride belongs to the class of organic compounds called acyl chlorides, bearing both a bromine atom and the reactive acyl chloride group at the two ends of a five-carbon chain. Its structure reads as Br(CH2)4COCl. It gets sold mostly as a clear to pale yellow liquid, often tightly capped in brown glass bottles to keep it out of light and moisture. The product finds buyers dealing with specialized organic synthesis — both academic and industrial — as well as those optimizing synthetic routes in medicinal chemistry or agrochemicals. A good supplier always emphasizes purity, since trace contaminants mean headaches in synthesis, especially at scale. Most researchers I know routinely scrutinize certificates of analysis before anything gets pipetted or poured.
Physical & Chemical Properties
The molecular formula for 5-Bromovaleryl chloride reads C5H8BrClO, with a molar mass hovering around 211.47 g/mol. The compound gives off a pungent, irritating odor — not one anyone looks forward to. Its boiling point tends to fall in the 211–213°C range, but watch for significant volatility and reactivity at lower temperatures, especially if any moisture sneaks in. The substance dissolves well in organic solvents like diethyl ether and dichloromethane, though it hydrolyzes fast when water gets anywhere near. I’ve seen first hand how easily these reactive molecules fume if the cap’s left off, spewing irritating vapors that become hydrochloric acid in moist air. Its density usually sits just over 1.45 g/cm3, which matters during transfers and separatory funnel work.
Technical Specifications & Labeling
Chemical suppliers ship 5-Bromovaleryl chloride mainly in volumes from a few grams up to kilograms, labeled with standard hazard statements and safety pictograms. Purity levels above 97% reign as the commercial norm, really reflecting what academic and industry chemists demand. Any reputable supplier lists batch numbers, storage requirements (cool, dry, away from sunlight), and recommended shelf life — typically six to twelve months if the container stays tightly sealed. All labels on regulated shipments carry UN numbers, proper shipping names (Corrosive Liquid, Organic, N.O.S., containing 5-Bromovaleryl chloride), and full hazard statements linked to eye, skin, and respiratory risks. Documentation from quality suppliers includes spectra and chromatograms backing up the listed purity.
Preparation Method
Making 5-Bromovaleryl chloride usually starts with 5-bromovaleric acid or its corresponding alcohol. The most common synthetic path involves treating the carboxylic acid with a chlorinating agent like thionyl chloride (SOCl2) or oxalyl chloride (COCl)2, which strips out water and swaps the -OH group with chlorine, generating SO2 or CO, and HCl as byproducts. This method provides a decent yield, and the side products vent away as gases or get scavenged out by careful evacuation. Researchers sometimes favor phosphorus pentachloride (PCl5) for small-batch transformations if they want to dodge thionyl’s fumes. Once isolated, the product often gets distilled under reduced pressure to keep it stable. Every chemist I know who deals with this class wears extra gloves, keeps proper ventilation on, and never leaves the reaction potty-mouthed without a neutralizing bath handy.
Chemical Reactions & Modifications
The real value of 5-Bromovaleryl chloride comes from its double reactivity. The acyl chloride end attacks nucleophiles, perfect for making amides, esters, and other derivatives in one sweep. You can treat it with an alcohol in the presence of a base and crank out a brominated ester, or react it with an amine to create a brominated amide, which makes a nice intermediate for more complex constructions. Synthetically, the bromine atom near the end can take part in nucleophilic substitution, especially if you want to introduce further groups — think azides, alkoxides, or even convert to amines. It’s a handy "handle" that enables stepwise construction of branched molecules or attaches additional chains on demand. In the right hands, this dual-reactivity gives a lot of shortcuts to structures that would require far more steps starting from plainer acyl chlorides.
Synonyms & Product Names
5-Bromovaleryl chloride appears in catalogs under several synonyms, reflecting its straightforward structure. You sometimes see it listed as 5-bromopentanoyl chloride, 4-bromobutanecarbonyl chloride, or simply as pentanoyl chloride, 5-bromo-. CAS Number directories identify it as 39591-19-8 for clarity. In European databases, it’s common to run across names like pentanoic acid, 5-bromo-, chloride, which says exactly what the molecule is in its formal version. I always check labels twice, because close names can mean a totally different bromination site or a straight-chain isomer, both of which can blow an experiment off course.
Safety & Operational Standards
Working with 5-Bromovaleryl chloride isn’t something to take lightly. It reacts violently with water and moist air, spitting out hydrogen chloride fumes that corrode not just your equipment but your lungs and eyes if you’re not careful. Splashes sting instantly and take time to heal. Everyone in the business wears snug-fitting goggles, full-protection gloves, and long sleeves, and only opens containers inside properly ventilated hoods. Material safety data sheets rate it as corrosive and acutely toxic by inhalation, and remind workers to keep neutralizing agents — sodium bicarbonate, for example — within reach for spills. Waste gets collected in Drums marked Corrosive, and nobody dumps anything down the drain without render-safe protocols. Labs operating in Europe or North America must document each use, track inventory, and verify that containers sit in secondary containment bins. Strong standards don’t exist only for compliance; they make sure nobody gets hurt and every experiment runs without nasty surprises.
Application Area
5-Bromovaleryl chloride means business for researchers and process chemists interested in creating custom molecules. Its ability to introduce a lengthened, bromine-bearing carbon chain makes it prized for building drug candidates, agrochemical leads, and specialized polymers. Medicinal chemists frequently select this reagent for constructing molecular frameworks that mimic natural products or display desired biological effects, especially when a bromine atom at the chain end supports further modification or acts as a label. It crops up in material science circles too, where the arising ester or amide products get built into advanced coatings or plasticizers. Academic research features it in tools to probe structure–activity relationships, as the bromine’s reactivity lets teams snap on azides, thioethers, or other labels for imaging or crosslinking studies. Regulatory filings and patent applications often cite it as an intermediate in multi-step routes to more high-profile targets.
Research & Development
The push to expand the chemistry possible with 5-Bromovaleryl chloride never really slows. Synthetic chemists keep looking for greener, less hazardous ways to manufacture it at higher purity, lower cost, and with less noxious waste. Some labs test out milder chlorinating agents that limit gas evolution or swap out liquid-phase reactions for solvent-free approaches using solid supports. Researchers in pharmaceutical development profile various acyl derivatives to tune drug metabolism, distribution, and clearance — the kind of groundwork that streamlines new drug launches. Industrial teams continue to optimize processes for scale-up, aiming at both energy savings and waste minimization. Literature reviews from the last ten years show an uptick in structural analogs, often with variations at the brominated position or the acyl moiety, which broadens the types of molecules achievable with stepwise additions. I’ve noticed the surge especially in patent filings related to novel antibiotics and next-generation herbicides.
Toxicity Research
Safety research leaves no stone unturned with a compound like this. Acute exposure, even by vapor, can damage mucous membranes and tissue. Animal studies show that inhalation or skin contact leads to rapid onset of symptoms — coughing, eye burns, and lasting rashes. Most data point to the corrosive nature rather than long-term cumulative effects, though any compound with both acyl chloride and bromine wakes up curiosity about genetic or developmental toxicity. Toxicology review panels usually recommend minimizing exposure using every engineering and protective control possible, reinforcing standard practices about fume hoods and full-face shields. What little chronic data exists suggests environmental persistence isn’t a massive issue since reactive hydrolysis happens fast, but any release into the environment gets flagged because byproducts can affect aquatic life. Even in clean, regulated labs, everyone respects the risk and never cuts corners with handling protocols.
Future Prospects
The demand for halogenated building blocks looks set to keep rising, which makes 5-Bromovaleryl chloride a likely choice for more advanced downstream chemistries. Pushes for cleaner synthesis — less reliance on nasty chlorinating agents, leaner waste footprints, and easier post-use treatment — drive innovation here. As biocatalysis and flow synthesis become more widespread, suppliers start tweaking formulations and packaging for plug-and-play compatibility with next wave reactors. From a commercial angle, new drug and crop protection pipelines constantly need intermediates that can carry reactive groups in, and the unique features of this compound — chain length and reactivity — fit the bill well. Regulatory pressure may steer production toward less hazardous conditions and improved operator safety, but the core market for brominated acyl chlorides seems likely to stay strong among those pushing the envelope of fine chemical science.
Understanding the Structure
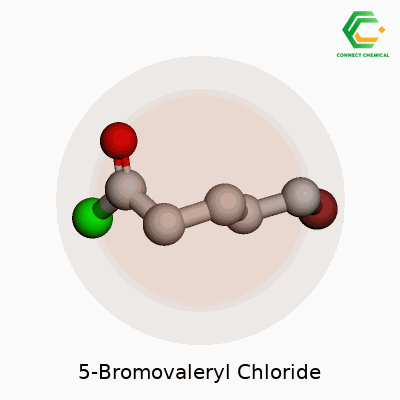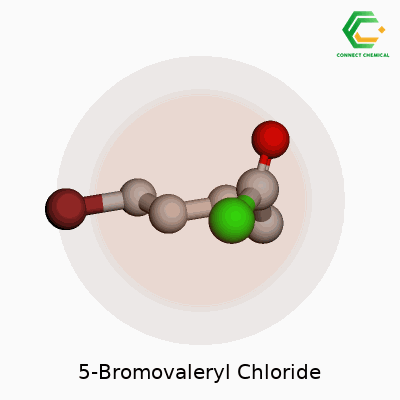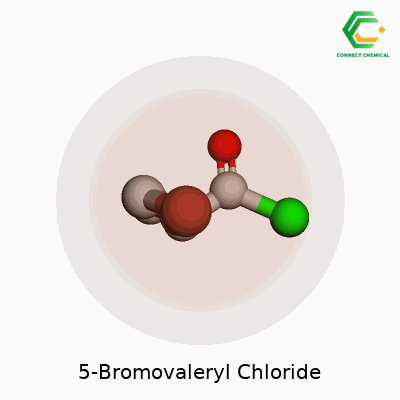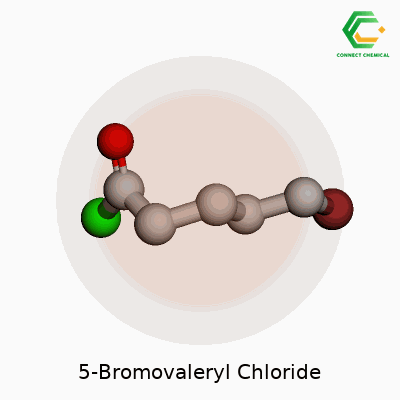
5-Bromovaleryl chloride has the chemical formula C5H8BrClO. To picture this compound, start with a five-carbon chain, known as a valeryl group. A bromine atom attaches to the fifth carbon, and the very end of the chain turns into an acyl chloride group. It might sound complicated at first glance, but drawing it out helps reveal its logic. Organic chemists appreciate molecules like this because small changes in structure lead to massive differences in reactivity and usefulness.
Why It Matters
In laboratories, compounds like 5-bromovaleryl chloride allow for targeted chemical transformations. Functional groups such as the bromine and chloride make it a versatile starting material for synthesizing more complex molecules. The pharmaceutical industry often works with these intermediates to build medications. In chemical research, this compound helps unlock new pathways to study reaction mechanisms or develop specialty compounds for advanced applications.
Handling and Hazards
Experiencing firsthand the scent and sharpness of acyl chlorides, it's clear that these chemicals need respect. 5-Bromovaleryl chloride, like other acid chlorides, reacts aggressively with moisture in the air. That reveals an important safety issue: proper storage and handling stop accidents before they happen. Direct contact can burn skin, and even small spills fill a lab with biting fumes. Using gloves, goggles, and fume hoods becomes a normal part of life around reactive chemicals. Anyone working with this material needs routine safety training and a real awareness of airflow and ventilation.
Environmental Impact
The rise of green chemistry highlights the need to rethink our approach to reactive chemicals. Disposal of acyl chlorides, especially halogenated types like this one, poses a challenge because typical waste streams can't neutralize the residues easily. Halogenated waste, once released, lingers in soil and water. Environmental scientists keep pushing for alternative synthesis routes that create fewer dangerous byproducts or employ safer reagents. Change comes slowly in the world of specialty chemical manufacturing, but strong evidence from toxicology studies keeps pressure on companies and researchers to improve protocols.
Improving Safety and Sustainability
Shifting from personal laboratory experience to bigger industrial practices, process engineers can redesign reaction setups for less waste. Containment systems and scrubbers handle fumes before they reach the atmosphere. Automated sensors spot leaks quickly, cutting down on exposure risks. Regulatory bodies like OSHA and the EPA watch chemical plants closely. Transparency and routine reporting keep companies accountable, but it also encourages investment in safer processes. Anyone supervising chemical manufacturing knows that small improvements, repeated and reinforced, lead to lasting safety gains.
The Role of Education
Chemistry students benefit from learning not just the formulas and reactions, but also the impact of chemical choices on health and the environment. My own undergraduate training made a habit of discussing both the practical and ethical issues posed by powerful reagents. Open discussion in classrooms, professional societies, and industrial labs raises the bar for safety and responsibility. Building a culture where people look out for one another and share best practices matters more than any single piece of equipment or protocol.
Summary
5-Bromovaleryl chloride, C5H8BrClO, serves a role in both research and industry, balancing promise and hazard. Paying attention to safe handling, exploring greener alternatives, and prioritizing education shape a healthier, more responsible chemical community.
Getting to Know 5-Bromovaleryl Chloride
5-Bromovaleryl chloride often finds its way into laboratories and production floors where creativity in chemistry happens. This compound pops up frequently in life science research, especially in projects working on pharmaceuticals, agrochemicals, and specialty materials. Anyone interested in the business of building new molecules will, at some point, run into this handful of a reagent.
Forging New Pharmaceutical Compounds
Drug development relies on reliable building blocks, and 5-Bromovaleryl chloride fits that bill. Medicinal chemists reach for it to create intermediates—those stepping-stones that get reshaped into active pharmaceutical ingredients. What makes it so useful is the bromine atom sticking off one end. Bromine makes further reactions straightforward: it’s easy to swap for other chemical groups, carving paths to more complex structures.There’s a reason you’ll spot this compound in scientific journals—they’re working out new antibiotics, antivirals, or sometimes entirely new molecular scaffolds for rare disease research. These advances depend on reagents that react cleanly and predictably, so labs can keep pushing the envelope on discovery and testing.
Agricultural Protection and Productivity
Agriculture has a huge stake in chemistry. 5-Bromovaleryl chloride lands in this industry because it helps build pesticides and herbicides with specific action on damaging pests or tough weeds. When companies look to tweak a molecule’s effectiveness or environmental persistence, this compound’s reactivity offers several routes to new analogs. The goal always stays the same: produce healthier crops, waste less yield, and target threats with fewer side effects on beneficial insects or the environment.
Chemical Synthesis—A Researcher’s Swiss Army Knife
You don’t need to be making medicine or farm products to get value from this compound. Organic chemists often use it as a go-to acylating agent. That means it delivers a piece of itself to a growing molecule, helping to tack on new architectures. Need a tailored keto group or a nifty five-carbon chain that’s ready for more attachments? This reagent does the trick.Sometimes synthesis isn’t glamorous, just practical. Researchers looking for a flexible route often keep compounds like 5-Bromovaleryl chloride close at hand—not just for flashy breakthroughs but for solving everyday problems in analytical chemistry or process development.
What Safety Tells Us About Responsibility
No serious chemist ignores the hazards with chemicals like this one. Exposure can cause burns or breathing trouble, so labs build in serious precautions: chemical fume hoods, protective gloves, and goggles as basic gear. Training matters here because a moment’s distraction can turn costly. Safe handling guidelines published by regulatory agencies don’t just gather dust—they shape how real people treat these reagents, day in and out.Tough rules protect researchers and the environment. Strict disposal rules make sure everything gets neutralized before leaving the lab. Companies invest in engineering controls as well as ongoing education for their teams, because nobody wins if a shortcut causes harm.This vigilance fits with how industries around the world look to balance innovation with concern for neighbors, clean water, and safe workplaces. With every batch of 5-Bromovaleryl chloride that ships out, responsibility travels right alongside.
Strengthening Connections in Innovation
Progress depends on reagents like 5-Bromovaleryl chloride. Those who know how to use it safely and smartly—armed with up-to-date facts, good habits, and the right materials—keep the wheels of discovery turning. This compound never stands alone; it connects research, industry, health, and safety. Treating these links with respect builds a foundation for better breakthroughs in science and technology, year after year.
The Stuff You Don’t Want On Your Hands
If you’ve worked in a lab, a bottle labeled “5-Bromovaleryl Chloride” stands out—and not in a good way. With every fume hood comes a sense that every chemical needs its own sort of respect, but some demand extra care. This one fits that bill. It’s known to be corrosive and reactive with water, so a standard laissez-faire approach invites trouble. Even a quick splash or opened cap without a mask can give you more than just a bad day. Skin, eyes, lungs—all are fair game for damage. That’s not something I risk, and no lab tech I know does, either.
Why Safe Storage Isn’t Just a Suggestion
I once watched a rushed coworker regret stacking incompatible bottles on the same shelf. 5-Bromovaleryl Chloride belongs in a cool, dry place, sealed up airtight. Any hint of humidity or water causes it to react, even releasing corrosive fumes. One whiff in a tight storage room and you’ll know something’s up. It prefers a chemical storage fridge, hidden away from anything amine-based or strong bases. Fume hoods and chemical gloves—nitrile or butyl—not the dime-a-dozen latex kind, provide the only decent barrier against spills. If I haven’t checked the gloves for tears beforehand, I’m outright gambling with my health.
Facts Back Up The Precautions
The Globally Harmonized System puts 5-Bromovaleryl Chloride into “Acute Toxicity, Category 3.” That means short-term exposure can do real damage. Symptoms hit fast—burning throats, tearing eyes, even burns on contact. If it escapes containment, it doesn’t just put handlers at risk, but everyone in the room. Flammable storage cabinets work best, preferably with secondary containment trays. I’ve seen the difference between trays and none—the aftermath with none looks like chaos, if not a complete shutdown of that part of the lab.
Simple Steps Work Best
It’s tempting to see warnings and figure that you’ll be fine “just this once.” No shortcut saves time if a spill happens. Labeling the container with the date and opening status gives everyone a heads-up. Small things like always using a pipette—not pouring—seem fussy, but they give control, drop by drop. I count on designated chemical waste streams for chlorinated acids, never regular lab trash. Emergency showers and eyewash stations become your backup plan, not the first line of defense. You’ll never meet a veteran chemist who skips testing the eyewash every session; it’s too risky not to.
Improving Safety Culture
It’s easy to rely on routine, but refresher safety training helps, especially for newcomers. Posting up-to-date material safety data sheets (MSDS) and step-by-step cleanup guides near storage sites keeps everyone honest. I’ve seen teams run through emergency drills with outdated info, and mistakes get made that wouldn’t happen with a quick review. Fresh air—forced ventilation—makes a huge difference, as does limiting how much you buy at once. A smaller bottle is easier to control and doesn’t sit unused as a hazard. Double-checking compatibility charts before any transfer or mixing saves headaches down the line. Most incidents I’ve heard about trace back to someone ignoring the basic rules. Experience reinforces: following the core steps keeps everyone safer and the rest of the operation running smoothly.
Everyday Risks
People deal with health hazards all the time, even outside industrial workplaces. Cleaning chemicals at home, pesticides in the garden, auto fluids in garages—all expose us to harmful substances. Just spending time around these products can irritate skin, eyes, or even airways. Sometimes, simple mistakes like using the wrong cleaner or forgetting gloves leave us with rashes or burning eyes.
Common Health Hazards
The risks differ based on the substance. Solvents carry fumes that hurt lungs and may trigger asthma or headaches. Strong acids or bases found in toilet cleaners and drain openers burn through skin, sometimes in seconds. Pesticides and herbicides can cause everything from dizziness to nerve issues. Even common bleach burns hands or triggers breathing problems if used without good airflow.
Over the years, I’ve seen how quickly things can go wrong. A friend once splashed bleach on bare skin by accident and got burns in minutes. At a job, I forgot to wear a dust mask while handling cleaning powder and spent the afternoon coughing. Workers in construction and factories often share stories of burns, coughs, or hives when handling chemicals without the right gear.
Routes of Exposure
Hazards get into our bodies through skin contact, inhalation, swallowing, and even eye contact. Fumes or fine dust hang in the air, entering the lungs with every breath. Children, who touch everything, often transfer things from skin to mouth faster than adults realize. Skin absorbs chemicals slower than lungs do, but even small splashes add up over time. Cuts or broken skin let chemicals in even faster.
First Aid Measures
Basic first aid makes a huge difference. For skin contact, get to running water right away and rinse for 15 minutes. The longer irritants stay, the worse the damage. Remove any clothing touched by chemicals. Never try to neutralize chemicals on skin with other chemicals—water is safest.
For inhaling fumes, get to fresh air quickly. Open windows, step outside, or switch on a fan. Persistent symptoms like coughing or dizziness call for medical help. Eyes are even more vulnerable. Flush with clean water for at least 15 minutes and avoid rubbing, because friction pushes the irritant deeper.
Swallowing chemicals, which often happens to kids, needs urgent attention. Avoid inducing vomiting unless poison control advises it. Sometimes vomiting worsens the harm. Dial emergency services or poison control. Bringing the chemical container to the doctor helps identify what went wrong.
Prevention and Awareness
Wearing gloves, goggles, and masks cuts the chance of injury. Keeping chemicals in original containers with clear labels stops accidental mix-ups—never store cleaning fluids in food jars. Good ventilation is key. Even simple fan use while cleaning saves lungs from fumes. Schools and workplaces need training about these risks. At home, locking away dangerous products keeps kids and pets safe.
Staying up to date with product instructions and latest safety information helps. People sometimes hold on to old habits or skip reading warning labels. Taking a moment to learn and share knowledge prevents lifelong harm. It always pays to have local emergency numbers and poison control contacts on hand—nobody expects an accident until it happens.
The Real Significance of Purity in Chemical Applications
Purity isn’t a dry statistic on a label. In the chemical world, the purity of a compound like 5-Bromovaleryl Chloride often determines the entire outcome of a project. For researchers and production teams, quality standards aren’t just paperwork—they affect results and safety. In my own experience, cutting corners on purity caused entire batches of products to fail analytical quality checks, setting back weeks of work. Most commercial sources provide this compound at a minimum purity of 97%, and sometimes up to 99%. That slight difference means fewer unexpected side reactions, better yields, and repeatable performance in subsequent applications. Labs working on complex syntheses—anything from pharmaceutical intermediates to specialty polymers—gain real trust in their results only when starting materials measure up.
Why Packaging Size Matters in a Working Lab
The available packaging size for 5-Bromovaleryl Chloride often reflects real-world needs. The standard sizes on the market are typically 5 grams, 25 grams, 100 grams, and 500 grams, though larger bulk orders can be arranged directly with producers. In academic research, a 5 or 25 gram package usually suffices, since many syntheses require small quantities to get a reaction going. In one of my past projects—setting up an undergraduate teaching lab—we intentionally bought only 25 grams to keep inventory manageable and avoid waste. At the industrial level, large-scale reactors chew through much more, so 500-gram bottles or even 1 kg drums show up. Overestimating needs leads to leftover material that loses potency or, worse, turns hazardous over time due to decomposition.
Practical Risks and Safety Concerns
5-Bromovaleryl Chloride comes with a pungent odor and reacts aggressively with water. Anyone who’s ever handled it knows the importance of tightly sealed packaging and solid labeling. Smaller bottles help minimize exposure and make it easier to use all the material before degradation sets in. In my work, we always opted for the smallest size that met our needs, which also meant less risk in case of an accident. Institutions focusing on safety regulations often encourage splitting shipments or using single-use ampoules when possible. Every chemist remembers the lessons from poorly packaged chemicals: ruined experiments and extra hazards that nobody wants.
Balancing Access and Quality Control
Not every supplier offers the same options, and not all storage environments guarantee the same shelf life. Sourcing from certified providers really counts. Suppliers with ISO certifications and transparent quality control records give buyers confidence. Once we started working exclusively with reliable vendors and specified the required purity and packaging size in our orders, delays and costly errors all but disappeared. Those small habits pay off when supply chains feel the pressure of rising demand or tighter regulations.
Paths Toward Better Lab Practices
Sophisticated labs and factories often get better at specifying their needs as soon as they understand how purity and packaging size affect efficiency. Training staff to recognize the difference between 97% and 99% purity pays off in both yield and safety. Sharing data with suppliers about real usage patterns encourages pack sizes that better suit reality. As I learned, keeping open communication between purchasers and the technical team can prevent buying bigger packs than necessary, which helps with both waste reduction and budget management. Focusing on the details—purity and packaging—turns out to prevent more problems than any emergency protocol ever could.