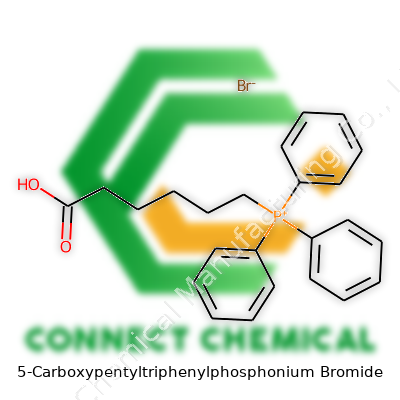
5-Carboxypentyltriphenylphosphonium Bromide: An In-Depth Commentary
Historical Development
Looking back on the journey of 5-Carboxypentyltriphenylphosphonium Bromide, it’s clear the compound grew out of a surge in interest around designing phosphonium salts with tailored functional groups. During the late 20th century, chemists chasing better mitochondrial probes and membrane-targeted agents homed in on triphenylphosphonium derivatives thanks to their reliable ability to cross lipid bilayers. Once researchers linked a carboxypentyl chain to the triphenylphosphonium core, the resulting molecule expanded the possibilities in both medicinal and analytical chemistry. Labs worldwide started using such molecules to uncover mitochondrial mechanisms, gradually building a track record for 5-carboxypentyltriphenylphosphonium bromide that sits deep in modern cell biology and organic synthesis.
Product Overview
As a product, 5-Carboxypentyltriphenylphosphonium Bromide shows up as a white to off-white crystalline powder—sometimes crystalline solid, sometimes a sticky solid depending on synthesis and humidity. This compound’s market draws from research circles eager for carrier molecules capable of ferrying tags or cargo across membranes. Suppliers catering to these customers typically list purity above 97%, stressing absence of residual solvents and heavy metals. Researchers and technical staff expect solid documentation, including a certificate of analysis for every batch, which ensures experiments remain reproducible and regulatory audits run smoothly.
Physical & Chemical Properties
This compound doesn’t shy away from demanding conditions. Dropping into a solution, the distinct crystal structure dissolves well in polar solvents like DMSO and methanol, thanks largely to its ionic bromide component and the polar carboxylic acid. In water, solubility varies with pH, shifting between the protonated and carboxylate forms—sometimes forming visible aggregates if handled clumsily. The melting point slides in around 180 to 190°C, telling you a lot about stability and handling needs during reactions. Unlike many unstable organophosphorus molecules, this triphenylphosphonium derivative stands up well to air and most bench-top conditions, though exposure to strong bases or acids over time will slowly break its structure down.
Technical Specifications & Labeling
Technical data sheets focus on molecular weight (about 462 g/mol for the bromide salt), CAS number, and full IUPAC name. Storage recommendations generally ask for conditions below 8°C, out of direct sunlight, in tightly closed containers to keep moisture away. Labeling requirements follow global standards—the GHS label shows clear hazard pictograms when relevant, with a concise statement about skin and respiratory irritation, as well as disposal guidance for environmental stewardship. Chemical suppliers make batch tracking simple, with batch numbers and expiry dates front and center. On the lab shelf, you’ll see hazard statements referencing eye and skin contact risks, along with emergency measures to contain and wash away accidental spills.
Preparation Method
Synthesizing 5-carboxypentyltriphenylphosphonium bromide takes solid practical chemistry skills. The starting material, typically 5-bromovaleric acid, gets mixed in an aprotic solvent with triphenylphosphine. Stirring this mixture under reflux makes the alkyl bromide react in a classic SN2 mechanism, giving that iconic quaternary phosphonium salt. Finishing the synthesis, you remove any organic by-products using repeated crystallization or column chromatography. For higher purity, careful drying under vacuum eliminates trace moisture, which often plagues storage attempts. Every qualified chemist checks yield, melting point, and NMR or HPLC spectra before any batch ships out for use— nobody wants an unknown impurity derailing a week’s experiments.
Chemical Reactions & Modifications
This compound steps up as a versatile intermediate in organic synthesis. In the lab, that carboxyl group opens doors to forming esters and amides with ease—using a carbodiimide or EDC, for instance, to attach peptides, fluorophores, or other molecular tags. The triphenylphosphonium moiety acts like a homing device, shuttling these modified molecules straight across mitochondrial membranes. Some labs have turned to coupling this phosphonium salt with activated NHS esters or alkynes for click chemistry. In redox studies, the compound’s structure stays intact in reducing environments, which lets researchers track probes or drugs in live cell imaging without worrying about premature degradation.
Synonyms & Product Names
Depending on the context, suppliers and researchers call this compound by a mouthful of names—5-carboxypentyl triphenylphosphonium bromide, pentanoic acid, 5-[(triphenylphosphonio)methyl]-, bromide, and plain old CPTPP Br. Each label traces back to the same backbone, so folks in pharmaceuticals or biotechnology all end up speaking the same language, even if catalog numbers differ between vendors. Some specialty chemical stores use brand or catalog names—sometimes with a dash, sometimes with “ionic carrier”—but real-world scientists always double-check with a structure diagram.
Safety & Operational Standards
Lab life teaches a healthy amount of respect for every bottle of 5-carboxypentyltriphenylphosphonium bromide. Direct skin or eye contact invites local irritation, which can be serious if someone works without gloves. Spills call for prompt cleaning with copious water before chemical disposal in line with institutional guidelines. FDA or OECD safety standards recognize the compound’s intermediate toxicity profile and generally require all work to happen beneath a certified fume hood. Those handling the gram-scale synthesis take added precautions against inhaling dust or introducing the powder into open wounds—phosphonium salts can act as cell penetrators in less predictable ways compared to less charged molecules.
Application Area
Where 5-carboxypentyltriphenylphosphonium bromide gets used, the molecule does real work. In targeted mitochondrial research, it shines as a targeting agent, ferrying biotin, dyes, or even metal chelators past cellular defenses right where scientists want them—inside the mitochondria. Drug delivery researchers appreciate the strong membrane permeability and use derivatives as models for new therapeutic carriers that could one day treat cancer or degenerative neurological conditions. Proteomics labs exploit the functionalized carboxyl group to build elaborate affinity probes, linking them to beads or surfaces looking for low-abundance mitochondrial proteins no traditional tool would catch. Diagnostic imaging also profits from the ability to send fluorescent or radiolabeled tags to specific organelles, lighting up sub-cellular habitats not easily reached with standard molecules.
Research & Development
Stepping into R&D labs in this area, the air buzzes with expectation. Chemists and biologists alike continuously modify the carboxypentyl chain, swapping in isotopes, shortening or lengthening the linker, or shifting to more robust anions—just to explore how each tweak affects the molecule’s performance. Groups at the intersection of chemistry and medicine set up in vitro and in vivo screens, tracking how these modifications impact cellular toxicity, bioaccumulation, and clearance kinetics. By collaborating with computational chemists, wet-lab workers model mitochondrial targeting before ever lifting a flask. This data-driven push keeps spawning knockout papers in pharmacology and analytical journals, feeding back into better reagents for the next wave of mitochondrial research tools or potential therapeutic prototypes.
Toxicity Research
Research on toxicity pulls double duty—protecting lab workers and driving future drug safety. Studies on 5-carboxypentyltriphenylphosphonium bromide often start with cell-culture models, confirming that the molecule disrupts mitochondrial membranes at high doses, sometimes slowing cell growth or triggering apoptosis, especially in non-cancerous cells. At the same time, this property makes it a valuable weapon for targeted drug strategies that seek to cripple diseased mitochondria in cancer cells. Rodent models raise flags about cumulative toxicity in organs with high mitochondrial content, like heart and brain, signaling the importance of careful dose selection and regular monitoring. Reviews in toxicology journals reference this dual-edged sword—high value as a model system comes with responsibility: clear safety data, well-controlled experiments, and full disclosure of batch properties before pushing new analogs into animal or human testing.
Future Prospects
Looking ahead, the future of 5-carboxypentyltriphenylphosphonium bromide ties tightly to demands for smarter therapies and ever more precise molecular imaging agents. Every new research project fuels innovation, from attaching prodrug forms to the carboxyl group, to developing rapid-screen diagnostics that home in on early markers of mitochondrial dysfunction. Real traction appears through partnerships between academia and industry, pushing the molecule beyond niche lab tool status into commercial therapeutics and diagnostics pipelines. Experts carve new research territory by engineering related phosphonium salts with multi-modal targeting or reduced off-target effects. Excitement also surrounds green chemistry efforts, searching for earth-friendly preparation reactions and scalable manufacturing that don’t rely on heavy metals or toxic solvents. As science keeps advancing, 5-carboxypentyltriphenylphosphonium bromide is bound to stay under the spotlight for years, shaping drug design and diagnostics at the floor of cell biology.
A Chemical With a Targeted Role
5-Carboxypentyltriphenylphosphonium Bromide doesn’t catch as much limelight as caffeine or aspirin, but its work in the research world drives important breakthroughs. The key detail that sets this molecule apart sits in its triphenylphosphonium group. Chemists latch onto that structure because it helps the compound pass straight through cell membranes. The whole process comes down to the positive charge on the phosphonium, which attracts it to the negatively charged mitochondrial matrix inside cells.
This property turns the compound into a special delivery truck for mitochondria-targeted drugs, probes, and imaging agents. I’ve seen years of effort go into developing treatments for diseases that start in mitochondria—like some neuromuscular disorders. Being able to get molecules exactly where they’re needed, deep inside a cell, takes out much of the guesswork. It isn’t just academic curiosity at work. The delivery systems built around triphenylphosphonium handle some of the most challenging problems in drug development.
Direct Impact in Research and Diagnostics
Lab teams use 5-Carboxypentyltriphenylphosphonium Bromide as a linker. Scientists can attach different molecules to its carboxypentyl side chain, and the phosphonium part guides the cargo into the mitochondria. I ran into this during work on reactive oxygen species, which are linked to aging and illness. With a fluorescent tag hooked onto the compound, you can actually see oxidative stress build up in real time under a microscope. This kind of imaging would be tough, if not impossible, without a delivery method like this.
Mitochondrial studies have pushed medicine forward. For example, researchers have tracked how certain drugs damage cell metabolism and how toxins trigger cell death. Without precision targeting, conclusions would be blurred by off-target effects. With this bromide salt, there’s a window into what’s really happening inside that powerhouse of the cell. One study from the Journal of Biological Chemistry showed how researchers tracked changes in mitochondrial membrane potential through tagged phosphonium compounds. Data like that turns into better screening tools for early signs of disease.
Sourcing and Lab Handling Matter
Some chemicals feel like commodities, but not all meet the bar for high-level science. 5-Carboxypentyltriphenylphosphonium Bromide arrives with strict requirements for purity. Impurities won’t just cloud results; they ruin months of patient planning. Any lab ordering the compound spends time checking certificate data and, often, running independent checks.
I’ve heard from experienced researchers about struggles with unreliable suppliers, which slow down projects and eat up grant money. Strong supplier relationships—based on transparency, solid track records, and documented quality controls—make all the difference. Labs also train their staff to store such chemicals away from humidity and light. Each step protects both staff and the insights they hope to find.
Roadblocks and Avenues Forward
Cost and availability limit broader research, especially for smaller labs. Although the chemical itself forms the backbone of much mitochondrial science, price hikes hit hard, and students must stretch budgets. Streamlined synthesis, backed up by open-access protocols, would boost access and cut costs. Collaboration among academic labs could help, through shared purchasing or in-house production.
Science marches ahead on the strength of tools that deliver—and 5-Carboxypentyltriphenylphosphonium Bromide has quietly powered countless breakthroughs by getting the right molecules to the right cellular addresses. As technology opens up more of biology’s black boxes, compounds like this one open doors in ways raw theory never can.
Getting to the Core: Counting Atoms and Elements
I remember the first time I tried to decode a big chemical name. The confusion over so many rings, side chains, and elements initially left me lost. With enough repetition, though, piecing the puzzle together gets easier—like charting out the building blocks of 5-Carboxypentyltriphenylphosphonium Bromide.
Break it down: triphenylphosphonium is a phosphorus atom bonded to three phenyl groups (benzene rings attached directly to phosphorus). Add a linear five-carbon chain (pentyl) with a carboxylic acid tail (carboxypentyl) stuck to the phosphorus. Top it off with a bromide counterion to balance the positive phosphorus center. The final molecular formula: C24H24BrO2P.
Why This Chemical Structure Pops Up in Research
Digging into molecules like 5-Carboxypentyltriphenylphosphonium Bromide helps chemists and biologists with all kinds of problems. The bulky triphenylphosphonium group isn't just for flash—it helps molecules slip into mitochondrial membranes in living cells, much like a key fitting a lock. Drug researchers value this skill. Mitochondria are the cell’s engines, and targeting medications to that engine often improves disease treatments, including promising cancer therapies.
The carboxypentyl tail does more than extend the molecule’s length. It brings potential for further reactions—attaching drugs, fluorescent tags, or other chemical handles. In the lab, researchers often use “click chemistry” to tether new components right at that carboxy group. This tiny flexibility turns the molecule into a springboard for new experiments.
Safety and Handling: No Skipping Steps
A stroll through any synthetic lab shows the importance of careful chemical handling, and something with a formula like C24H24BrO2P is no exception. Bromide salts call for gloves and goggles. Phosphonium salts, especially in powder form, don’t forgive carelessness. Accidental spills—common during hasty weighing—stick to surfaces and call for diligent cleanup. Good ventilation and solid protocols matter, even on days when the pressure to get results stretches patience.
Supply Chain Realities and Testing for Purity
Fellow researchers sometimes grumble about the hassle of getting specialized chemicals. Sourcing a pure 5-Carboxypentyltriphenylphosphonium Bromide batch can stretch the patience of even veteran chemists. The price fluctuates with market demand and source country. Shipping delays or customs holdups can tap the brakes on a project—even a simple synthesis can grind to a halt without one key ingredient.
Quality testing isn’t optional. Supply houses promise >95% purity, but running your own NMR, mass spectrometry, or IR checks becomes second nature. Even a stray contaminant can derail an experiment or lead to bad data. In years of bench work, I’ve seen more ruined weeks due to poor-quality reagents than failed reactions.
Improving Availability and Research Progress
Better international collaboration might help smooth logistics, but on a smaller scale, more sharing between academic labs would help cut costs. Open sharing of protocols, data, and best suppliers could save money and keep discovery on track. I’ve seen the difference first-hand in lab groups plugged into global research networks versus those working in isolation.
Molecules like 5-Carboxypentyltriphenylphosphonium Bromide don’t just fill a catalog—they speed up science. Cracking the formula, knowing the risks, and splitting information with others brings a better shot at tackling real-world problems such as mitochondrial diseases and targeted drug delivery.
Understanding What’s at Stake
Handling specialty chemicals like 5-Carboxypentyltriphenylphosphonium Bromide makes a difference in both scientific progress and workplace safety. Forgetting to review a material’s specific storage needs can put research, equipment, and most importantly, people at risk. Before touching a single container, I always scan the Safety Data Sheet and double-check any updates from reliable chemical suppliers or professional bodies like the American Chemical Society. These aren’t minor steps; I’ve seen firsthand what a leaky cap or a poorly labeled container can do when mixed with humidity or light.
Temperature and Moisture Matter
This compound keeps its integrity at room temperature, but only in a dry environment. Moisture is trouble, especially with organic salts. A little water vapor or a spill on a benchtop can begin to degrade its quality and mess up experiments. If the cap is even a little loose, it’s enough to let air in, and soon the purity drops off. Sealing containers tightly, using desiccators, and staying vigilant about spills save time and research money.
Light: The Silent Enemy
Some labs tuck chemicals away in clear glass jars, but that’s an open invitation for trouble with light-sensitive compounds. Over months of daylight, certain chemicals yellow or break down, which throws off results. Storing this one in amber glass containers or a dark cabinet helps maintain purity and extends shelf life. I never leave these sorts of bottles near windows or lab benches exposed to sunlight, no matter how rushed the day gets.
Labeling Sets the Standard
Accurate labeling looks simple, but it’s one of the strongest defenses against accidental mixing and misuse. I learned early that scribbling something quickly isn’t enough. Every bottle on my shelf gets labeled with the product’s full name, the date received, and the expiry date. If there’s a hazard—say, skin or eye irritation—it goes right on the label. This level of detail means no guesswork when colleagues or I grab materials, even months later.
Avoiding Contamination
Cross-contamination sneaks into a lab routine if people get too comfortable. Clean scoops and spatulas, single-use gloves, and never dipping into containers with dirty instruments—that’s habit now. I set aside a dedicated area for chemicals that interact badly with water or air, keeping them away from acids, bases, and solvents. Keeping work surfaces uncluttered not only feels better, it also heads off problems before they grow.
What to Do in Case of Spills
Preparedness isn’t just for emergencies. If 5-Carboxypentyltriphenylphosphonium Bromide spills, the right spill kit makes cleanup faster and safer. Plenty of labs keep kits on hand, but they only work if everyone knows how to use them. Training matters—I’ve run drills with colleagues so nobody freezes or panics if a bottle ever tips over. Ventilation is critical too: wearing protective gear and handling the powder inside a fume hood can save your skin, eyes, and lungs from exposure.
Staying Informed Means Staying Ahead
The science behind handling chemicals like 5-Carboxypentyltriphenylphosphonium Bromide keeps moving forward. I keep up with reputable journals and networks for updates or unexpected incidents. Relying on old habits alone leaves holes; ongoing education and checking in with peers who work with the same materials help fill those in. Doing things by the book isn’t about being formal—good storage practices protect research, equipment, and people, so every detail counts.
Chemical Safety in the Lab
Working with chemicals like 5-Carboxypentyltriphenylphosphonium Bromide isn’t about trusting that a chemical is safe just because its name sounds complicated. My own years in chemical research showed me that ignoring basic safety protocols rarely works out for the best. This particular phosphonium salt doesn’t exactly top lists of everyday hazards, but most lab veterans reach for their gloves and goggles without hesitation anyway. Not only does it contain bromide, a compound that can be tricky with prolonged exposure, but the triphenylphosphonium group sits in a family known for bioactivity—which can bring benefits in a medical setting or unexpected surprises for an over-confident bench chemist.
The Hazards Under the Hood
Rich academic literature shows that triphenylphosphonium compounds can punch through cell membranes. In research, that’s useful for targeting mitochondria, especially in studies about neurodegenerative disease. But this very property raises a flag for personal safety. Chemicals that can slip into cells with ease don’t just ignore cell types or species. If a compound can penetrate mitochondria—basically the engine rooms of our cells—it only makes sense to treat it with respect.
Looking through available safety data sheets for 5-Carboxypentyltriphenylphosphonium Bromide, basic cautions stand out: avoid inhalation, skin contact, and ingestion. It’s a white powder, which can spread in the air more easily than a chunky solid or sticky liquid. If it gets onto your hands, there’s a real shot it might cross the skin or linger until someone touches their face or food. Swallowing phosphonium compounds isn’t wise, either. With skin or eye contact, many users report irritation, redness, or allergic reactions. Even if longer-term data are thin, the absence of known disasters doesn't mean uncontrolled exposure is safe.
Why Risk Management Matters
I learned the hard way that being cavalier about chemical hygiene in shared spaces can bring trouble for entire teams. Lab stories circulate about cross-contamination putting sensitive projects and people at risk. If this bromide builds up in the body over days or triggers mitochondrial stress, effects might not show up immediately, making it easy to downplay the threat. Yet, one uncontrolled spill or dust cloud can create longer-term issues—think lab animals, air filters, or even personal health a year later.
Smarter Ways to Work Safely
Chemical companies and workplace regulators post clear instructions for a reason. Gloves designed for chemical work and snug-fitting lab coats block most accidents before they start. Standard vented enclosures like fume hoods keep fine powders away from noses and mouths, plus they keep powder off surfaces where it can be tracked home. It’s worth keeping good documentation, both for your own safety and for anyone who might handle the same material after you. Routine hand washing and quick spill cleanups drop overall risk even further.
If you’re keeping materials long-term, secure storage in labeled, sealed containers prevents accidental exposure later. Sharing clear hazard information with team members and new lab members helps everyone steer clear of big mistakes. It shouldn’t take a high-profile incident or regulatory scare to push for better practices.
Looking Ahead
My own rule: treat new or rare chemicals as though they’re hazardous, until research shows otherwise. This approach hasn’t failed yet. With regular risk assessments, up-to-date material safety data, and common-sense personal protective gear, hazards from 5-Carboxypentyltriphenylphosphonium Bromide and chemicals like it tend to stay under control.
The Science Behind the Compound
5-Carboxypentyltriphenylphosphonium bromide stands out as a phosphonium salt that bridges classic organic chemistry and work in modern biology. Featuring a triphenylphosphonium group, a five-carbon chain, a carboxylic acid tail, and paired with a bromide counterion, its structure gives several clues about how it dissolves in various environments.
How This Molecule Behaves in Water
Add a handful of 5-carboxypentyltriphenylphosphonium bromide to water. The carboxyl group gives this compound some hydrophilic character. This tail loves to reach out to water molecules, in contrast to the chunky aromatic rings, which tend to avoid a watery crowd. From what peers in biochemistry circles and lab life report, the compound shows moderate water solubility. You don’t see it vanishing instantly, but with stirring or a little heating, it goes into solution. The bromide anion lends a hand, carrying some ionic nature, which also encourages water to play along.
Interaction with Organic Solvents
Slide over to organic solvents. Toss it in dichloromethane or acetonitrile. The triphenylphosphonium moiety and the long hydrocarbon chain assert themselves. Organic solvents such as DMSO or methanol will take up this salt eagerly. Researchers rely on these solvents, especially in sensitive cell biology work, because many cationic phosphonium salts, this one included, move smoothly into solution. Solvents more on the hydrophobic side, like chloroform, also host this molecule with little trouble due to the aromatic bulk and flexible aliphatic chain.
Solubility Challenges and Real-World Examples
Not every application works at the same scale. Sometimes, labs try to load high concentrations into buffer systems, especially when targeting mitochondria with triphenylphosphonium-based probes. The balance tips quickly—a little too much salt, and the solution clouds up. If you ever prepped some mitochondrial stain, you probably noticed a fine line dividing a clear solution from a stubborn precipitate. It helps to dissolve the compound first in DMSO, then dilute into water, letting each solvent handle the part it's best at. That’s a trick many researchers pick up along the way. In phosphate-buffered saline or cell culture medium, limit the concentration to keep everything dissolved and bioavailable.
Why Solubility Matters—And How to Improve It
Without the right solubility profile, work with cellular imaging or targeting drugs to mitochondria doesn’t get off the ground. Precipitation leads to loss of accuracy and wasted sample. Some chemists look to tweak the structure, adding substituents that pull in more water. Others optimize their solvent mix, using a touch of ethanol or DMSO before finalizing in aqueous buffer. Filtering freshly made solutions catches small particles and avoids performance swings. Documenting concentration limits in protocols boosts reproducibility for everyone, from grad students to senior researchers.
Evidence and Experience Inform Solubility Choices
Peer-reviewed data reinforce these tips. The literature ties high water solubility to introduction of polar groups or careful counterion selection. In practice, blending solvent choices—starting with a strong organic and diluting—preserves molecular function while dodging the pain of precipitation in biological work.
Moving Forward with Confidence
Every experiment poses a new question about solubility, and 5-carboxypentyltriphenylphosphonium bromide is no exception. Chemistry and biology both benefit from trial, error, and well-documented routines. Keeping an eye on solvent composition, staying within proven concentration limits, and sharing outcomes all push the science ahead and keep precious solutions clear—literally and scientifically.