8-Aminooctanoic Acid: A Detailed Look
Historical Development
The path of 8-aminooctanoic acid stretches back to the early curiosity within biochemistry labs in the mid-twentieth century. Researchers looked for new building blocks with promising features, and among various chain-length omega-amino acids, the eight-carbon member stood out. In the 1960s, increased attention landed on this molecule because industries began shifting toward advanced plastics and nylon variants. A dedicated push in synthetic chemistry helped refine the isolation and production of this compound. As scientists tuned catalytic hydrogenation and advanced alkylation, more efficient synthesis routes emerged, shaping not only the compound’s purity but its pricing and availability, as experienced today.
Product Overview
8-Aminooctanoic acid serves as more than just another specialty intermediate. Its structure—an eight-carbon saturated chain terminated by an amino group on one end and a carboxyl group on the other—means it slips neatly into polymer science, peptide design, and surfactant development. Decades spent studying its reactivity, solubility, and chain flexibility have shown it can pivot between engineering plastics, textile additives, pharmaceutical discovery, and even niche agricultural applications. Laboratories have adopted it for both large-batch production and small-scale, high-purity synthesis depending on research goals. These capabilities, in my experience, give research chemists and technical leads plenty of room for creative exploration.
Physical & Chemical Properties
8-Aminooctanoic acid appears as an off-white crystalline powder with a faint, amine-like smell. Chemically, the molecule carries the formula C8H17NO2, weighs 159.23 g/mol, and features a melting point within the range of 274–276 °C (around the melting points seen for similar alpha, omega-amino acids). It shows mild solubility in water and greater solubility in polar organic solvents—classic traits for medium-chain amino acids. Under normal storage, it does not absorb moisture rapidly but does best in a well-sealed container away from light and oxidizers. Slightly basic in aqueous solution, it can be driven to form salts readily with mineral acids. These qualities hold practical value for anyone balancing stability with the need to dissolve or react the acid efficiently in industrial or academic settings.
Technical Specifications & Labeling
Manufacturers usually offer this compound with purity above 98%, stabilized and dried for ease in transport and storage. Labels on technical-grade containers list not just purity, CAS number (3452-97-9), molecular weight, and batch number, but also trace residual solvents or contaminants to guarantee quality and reproducibility. For sensitive research fields, certificates of analysis extend to include heavy metal content, microbiological load, and spectral fingerprints (NMR, HPLC, IR). This attention to detail serves those of us in regulated industries, where one off-spec shipment can set a whole campaign behind schedule or throw off analytical results.
Preparation Method
Early approaches revolved around alkylation of glycine or hydrogenation of 8-cyanooctanoic acid. Today, most chemical suppliers rely on multi-step synthesis beginning with octanedioic acid, converting it to the corresponding amide and then transforming the amide group to amine under reducing conditions. Catalysts like Raney nickel or platinum assist in this transfer, limiting by-product formation. This process balances cost, yield, scalability, and environmental load. In some facilities, continuous-flow reactors have replaced classic batch systems to further tighten control over exotherms and improve throughput. These refinements have improved consistency and batch-to-batch purity, both crucial for commercial partnerships demanding tight tolerances.
Chemical Reactions & Modifications
The molecule’s linear structure and dual functional groups lend themselves well to derivatization. Chemists have acylated the amino group, esterified the carboxylate, or even linked the core chain into macrocycles for materials with enhanced mechanical properties. As a building block, 8-aminooctanoic acid readily enters amidation and peptide coupling reactions, expanding its use in biomimetic chemistry. It shows promise in preparing polyamide chains longer than those formed from shorter amino acids. In one organic synthesis campaign, I witnessed its modification with sulfonate groups, granting water solubility and surfactant behavior far better than what straight-chain fatty amines could achieve. Research teams have also experimented with incorporating aromatic or halogen substituents, adding handles for further elaboration.
Synonyms & Product Names
In literature and commerce, this compound goes by several names: octamethylenediamine monoacid, 8-amino caprylic acid, and octanoic acid, 8-amino-. Alternate listings include 8-aminocaprylic acid or 8-aminooctanoate. International suppliers may use local translations, especially throughout Asia and the EU, but the CAS registration or an unambiguous molecular formula typically settles any confusion during cross-border trade.
Safety & Operational Standards
All handling of 8-aminooctanoic acid calls for respect, despite its moderate toxicity compared to some shorter-chain analogs. Standard safety data points include mild skin and eye irritation risk, and potential respiratory discomfort if handled as a powder without proper ventilation. Many chemical hygiene plans treat it on par with non-volatile organic solids: wear gloves, work under a fume hood, don’t eat in the lab, and label any waste for hazardous collection. On large scale, strict containment helps reduce release risks, especially since amino acids can feed into bioactive by-products. Responsible producers comply with REACH, TSCA, and other chemical control regulations. Having personally audited a pilot plant installation, I can attest that training and routine monitoring often stop small exposures from ever becoming big problems.
Application Area
Industrially, 8-aminooctanoic acid often enters nylon-8 production, where it polymerizes to produce fibers with greater flexibility than classic nylon-6 or -66. Engineers see value in its melting point and tensile properties for specialty yarns and films. Pharmaceutical researchers use the free acid to assemble tailored peptides, sometimes seeking longer, flexible chain spacers between charged residues. The agricultural sector has evaluated its role as a dispersant and a functional part of advanced fertilizers. Synthetic surfactant manufacturers have modified its amine to tune hydrophilic-lipophilic balance, targeting everything from cleaning agents to emulsifiers. Several colleagues from my graduate studies found it useful in preparing modified cyclodextrin molecules, which help solubilize poorly soluble drug candidates.
Research & Development
Ongoing projects at universities and R&D labs focus on tapping 8-aminooctanoic acid for new material classes. There’s a surge of interest in biocompatible polymers and hydrogels for drug delivery or wound repair, using its straight chain to control molecular permeability and flexibility. Drug screening teams have started investigating phosphorylated and other derivatized forms as prodrugs offering measured release. Patenting activity now includes not just bulk nylon precursors but nanoengineered scaffolds and cross-linked gels. Investment pours in because chemists can blend the classic virtues of this amino acid—low toxicity, synthesis accessibility—with modern design thinking. In collaborative spaces, I’ve seen it spark debate on sustainability, especially as waste streams can be managed with less environmental impact than with industrial amines of higher toxicity or volatility.
Toxicity Research
Animal studies demonstrate that 8-aminooctanoic acid causes only minor acute toxicity by oral and dermal exposure, and the compound doesn’t build up substantially in tissues. Chronic studies remain limited, especially as new downstream uses roll out. Government agencies continue to push for clear labeling and traceability, and academic collaborations with public health programs collect emerging data. Some evidence suggests that metabolites might interfere with fatty acid oxidation in rare cases, though at levels far above those encountered in consumer goods or professional laboratories. Lab-based experience consistently shows that basic precautions—ventilation, protective clothing, and good storage practices—limit occupational risk. Most regulatory bodies place it in a lower hazard category than short-chain amines or halogenated analogues.
Future Prospects
Looking forward, the trend lines for 8-aminooctanoic acid remain positive. Demand in specialty polymers aligns with a broader move toward tunable, high-performance materials for medical devices, electronics, and low-impact packaging. Academic partnerships encourage greener synthesis and recycling protocols that could further reduce environmental footprints. There’s a push to expand its use in bio-inspired polymer networks and responsive materials, roles that suit its easy reactivity and moderate cost. In the pharmaceutical sector, its length and functional groups open possibilities as a linker for targeted therapy agents and as a backbone for hybrid nanostructures. Technological and regulatory advances—drawing from both past lessons and future needs—should help unlock even more innovative uses while keeping health and safety standards front and center.
Finding 8-Aminooctanoic Acid in Industry
People might not run into 8-aminooctanoic acid at the grocery store or pharmacy, but it is a backbone in modern plastic and polymer manufacturing. Known to chemists as a core building block for nylon-8, this compound helps turn raw chemical feedstocks into strong plastic fibers. Factories mix and match molecules like 8-aminooctanoic acid to create specific plastics for sportswear, carpets, and industrial fabrics. Each characteristic—stretchiness, durability, resistance to chemicals—relies, in part, on the smart use of this amino acid during the production process.
Why Nylon Needs This Compound
Big chemical plants value 8-aminooctanoic acid for its ability to make long carbon chains that link up tightly. For someone who has watched a spool of nylon thread at a textile mill, it’s clear how stringing together repeating molecular units delivers more than just shine. These fibers resist tearing. They don’t fall apart when exposed to oil or light cleaning products. Companies get reliability and strength, useful in carpeting built for high-traffic areas or clothes designed for repeated use.
Hidden Uses Outside the Textile World
8-Aminooctanoic acid doesn’t just have a job in making fibers. Some researchers found ways to work it into coatings and finishing treatments. Imagine metal parts protected from moisture or household appliances able to shed grime more easily. The same properties that help create tough plastic threads make coatings less likely to chip or crack. In the lab, specialty chemists experiment with this molecule to create sensors and smart drug-delivery systems, although real-world application in medicine still requires further testing.
Factoring in Environmental and Health Questions
Anyone who’s read about microplastics in the ocean or plastic waste on the news has thought about impacts long after manufacturing. Plastic fibers made with 8-aminooctanoic acid last a long time—sometimes too long for comfort. Waste management systems struggle to recycle specialty nylons in some regions, so the push grows for greener pathways. Factories experiment with bio-based production, and some research teams look at creating biodegradable versions. Evidence from studies—like the 2018 UNEP plastics report—shows roughly 300 million tons of plastic are produced yearly. That scale calls for chemistry with responsibility stitched in.
Safer Workplaces and Smarter Regulation
Chemicals that speed up manufacturing can pose risks for workers. Handling 8-aminooctanoic acid requires training and protective equipment. In many countries, workplace safety inspectors test air and keep tabs on how waste gets handled. Anyone who has worked on a factory floor will remember safety briefings about chemical spills or skin contact. Factories install fume hoods, provide gloves, and train staff how to clean up. Smart regulation means defining safe levels and making sure nobody gets left guessing about what’s in the air or water nearby.
Looking Forward: Better Plastics, Less Pollution
The future of 8-aminooctanoic acid hinges on blending high-performance materials with care for the planet. Industry moves quicker these days to find better ways to produce and recycle specialty plastics. Newer research focuses on closing the loop: designing fibers that can get reused instead of thrown away. For those invested in durable products—engineers, medical researchers, or even someone just buying a winter jacket—this amino acid’s story points to a tough balancing act, marrying performance with environmental stewardship.
Understanding 8-Aminooctanoic Acid
8-Aminooctanoic acid comes up in labs working with specialty chemicals, polymer science, and research chemistry. Most folks dealing with amino acids have some experience reading safety data sheets and following the usual rules: goggles, gloves, decent ventilation. This molecule, though, adds a wrinkle or two thanks to structure and potential hazards.
Potential Risks and Common Exposure
8-Aminooctanoic acid shares some traits with more familiar chain amino acids but doesn't show up in food or supplements. Handling it in powder or solution means thinking about dust particles and splashes. My own run-ins with similarly structured compounds taught me not to underestimate a mild-sounding chemical—fine powders sneak through the gaps in cheap gloves, and the skin around your eyes is a lousy place to discover a stinging residue.
Most amino acids under normal conditions don’t leap off the bench to cause big problems; still, that status never justifies being careless. The safety data published by manufacturers and compiled in open databases shows no evidence of 8-aminooctanoic acid being acutely toxic at modest doses. Tests in rodent models put the LD50 (lethal dose for 50 percent of test subjects) at several grams per kilogram—on par with other amino acids not known for high toxicity. By comparison, a sprinkling of table salt at high enough doses can cause more trouble.
Long-Term Handling and Lab Practices
Anyone handling it on a regular basis should stay cautious. Chronic exposure, even to low-toxicity compounds, sometimes leads to allergic skin reactions. The best habit involves putting on nitrile gloves, washing hands, and swapping out contaminated coats before lunch breaks. In my experience, too many people get casual after a couple months, right up until someone brings their hands to their mouth without thinking.
Workspaces benefit from routine—wiping benches, labeling every bottle, and setting up local exhaust if powder becomes airborne. I remember a chemist dismissing the need for a mask while weighing a powder, and two weeks later the department safety manager made masks mandatory after an uptick in mild respiratory complaints. Had the handling been tighter from the start, there'd have been no drama.
Enforcing Safe Protocols: Responsibility and Benefits
No law or guideline beats common sense layered over proper training. Whether at a startup biotech firm or a state university lab, the expectation should be that nobody handles powdered amino acids without knowing how to read a hazard label and find a nearby eyewash station. This isn't because 8-aminooctanoic acid threatens life and limb—it’s simply the only way to avoid those rare but still possible trips to the ER after a careless spill.
Some suppliers now offer safety webinars and print the most important warnings in plain English on their containers. This shift helps, especially for students new to organic synthesis. Open, honest conversations about near-misses or close calls, rather than sweeping them under the rug, make an enormous difference. After one too many harmless but scary splashes, a colleague started a group chat for reporting even minor incidents anonymously, and accidental exposures dropped sharply.
Concrete Steps toward Safer Handling
Lab managers should audit their spaces every few weeks and check that personal protective equipment remains in good shape. Signage in clear view beats a densely worded MSDS file tucked in a binder. Any time training new team members, I crack a few jokes about worst-case scenarios—humor keeps people alert without scaring them off from science.
8-Aminooctanoic acid won’t stop chemical research in its tracks. As with most things, smart habits, up-to-date training, and open conversations keep people safer than any technical document ever will.
The Building Blocks Behind 8-Aminooctanoic Acid
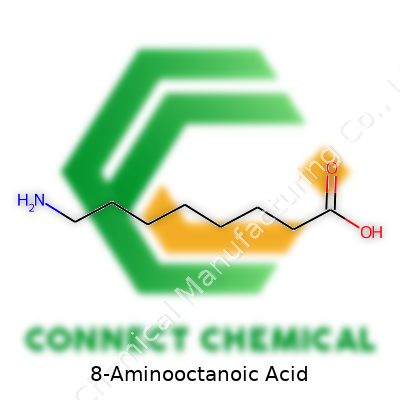
8-Aminooctanoic acid brings together eight carbon atoms, lined up in a chain. At one end, you get a carboxylic acid group (-COOH). On the other end, an amino group (-NH2) stands out. The rest of the carbons, six of them, fill the gap between these two parts. This means the backbone looks like this: NH2-CH2-CH2-CH2-CH2-CH2-CH2-COOH. Most chemists write its molecular formula as C8H17NO2.
The first time I read about 8-amino acids, I pictured long molecular chains acting like a highway. One functional group at each end means this compound is like a versatile tool, ready to click into other reactions. Synthetic chemists count on these features in the lab, especially when tweaking structures to form new polymers, such as nylon-8.
Why Structure Matters
The spacing between the amino group and the acid group changes how these molecules behave. In my experience, shorter chain analogs of amino acids, such as glycine or beta-alanine, bring the two groups closer together. That tight distance leads to different folding and reaction tendencies. In the case of 8-aminooctanoic acid, the seven carbons between the groups stop them from easily reacting with each other. This increases the likelihood that the molecule stretches out or lines up neatly, excellent for making polymers that don’t bunch up.
Scientists have counted on fatty omega-amino acids like this one to develop materials with flexible properties. The eight-carbon chain brings more distance, letting the molecule act like a mini rope for larger, tougher structures in the final material. These extended molecules support both strength and some stretchiness, important for fibers and plastics you tug and pull on in daily life.
Real-World Relevance
Understanding this structure proves crucial outside the lab too. I once helped with a project on biodegradable plastics. We spent long days hunting for molecules that could do the job—strong, flexible, and safe when they eventually break down. 8-Aminooctanoic acid offered a new pathway. The chain length contributed just enough flexibility, while the amino and acid groups served as handy handles that made the compound reactive with other partners. No single magic bullet exists in this field, but these little structural choices stack up fast when you want earth-friendly plastics that workers can actually produce at scale.
Supporting Innovation Through Deeper Chemical Knowledge
The push for sustainable chemistry today relies on understanding why chain length and functional group placement shape a molecule’s role. Background knowledge comes from decades of scientists mapping out what works—and what falls flat—when making strong, safe, new materials. Data from polymer science backs up this need for smart structural picks. For example, materials based on longer-chain amino acids can outperform those with shorter or bulkier structures, especially when durability and flexibility both matter.
Better knowledge supports better decisions. Chemists, engineers, and designers find more creative uses for simple compounds when the basic structure is clear and the limitations are honest. Whether working toward more sustainable packaging or lighter car parts, 8-Aminooctanoic acid’s unique combination of a long, straight hydrocarbon chain capped with active amino and acid groups earns its place in the toolkit. My own work showed me just how much every little detail in a molecule defines its future uses—and its real-world impact.
Understanding the Science and Risks
8-Aminooctanoic acid sits on the shelf in quite a few research labs and industrial facilities. On paper, it looks pretty straightforward—a solid, white powder with a simple molecule. Real-life handling tells a different story. Anyone with time logged in a lab knows chemical safety extends way beyond a data sheet.
This compound brings value in organic synthesis and polymer production. It also presents typical risks: dust inhalation, skin and eye irritation, possible reactivity under certain conditions. I’ve watched people overlook “simple” organic acids and pay for it later—red, runny eyes, cracked gloves, sometimes worse. People matter more than convenience. A slip here invites real trouble.
Storage Conditions: Staying Out of Harm’s Way
Cool, dry, and well-ventilated—those three words have saved more than one experiment and more than a few folks’ health. Moisture or heat lets degradation sneak in and can even set off unwanted reactions. Years of lab work prove that chemical shelf life isn’t imaginary. Push those limits and you only line up more risk.
This means putting 8-aminooctanoic acid in a tightly sealed container, preferably glass or a plastic with solid chemical resistance. Cabinet labeling ought to be clear and ugly—no excuse for reaching for the wrong jar with gloves on. Anyone who’s swapped one white powder for another knows the cleanup headache, not to mention the paperwork if a mistake hits the process line.
Every respectable storage area avoids direct sunlight. Light beats down on sensitive compounds and can shift a chemical profile inch by inch. My first boss always stashed amines on the lower shelf—keeps them away from the heat that rises and the risk of accidental spillage into eyes. Little habits help.
Contamination and Cross-Reaction Prevention
I once watched a fellow tech store a strong acid near a container of amino acids. All it took was a bit of condensation, a cracked cap, and both chemicals became contaminated. That mess forced us to toss out the batch. Chemical segregation in storage isn’t about bureaucracy. It means acids and bases don’t end up together, solvents stay capped, and everything’s properly labeled.
Avoid storing 8-aminooctanoic acid near volatile oxidizers or reactive metals. Keep inventory logs honest. I grew up hearing “write it down, double-check it later.” Lists catch what memory misses and can prevent those long, tense calls to safety teams.
Personal Safety and Emergency Planning
Storing a chemical the right way loses its value without the right PPE nearby. Safety goggles, gloves, and lab coats don’t belong in a locked closet. Anyone who handles chemical storage ought to know where the emergency shower stands, and fire extinguishers better not be buried behind boxes. The single worst chemical spill I witnessed grew ten times worse because someone tried to mop up with the wrong material. Training and forethought shape how safely a chemical sits on a shelf.
Room for Improvement: A Culture of Safety
Open discussion and routine inspections turn good intentions into real-world protection. Digital logs and storage alarms now replace hand-written lists in many labs. These upgrades offer alerts for open containers, expired batches, and temperature changes. Not everyone trusts automation, but a multi-layered system backs up human memory and keeps problems from growing unnoticed.
Respect for even “basic” chemicals lowers accident rates and builds a trustworthy lab culture. 8-Aminooctanoic acid deserves no less thoughtful handling than any other compound. Every smart safeguard keeps the work running smoothly and everyone a little safer for tomorrow.
A Closer Look at 8-Aminooctanoic Acid
8-Aminooctanoic acid might not roll off the tongue, but it’s a building block with a big impact, especially in the field of specialized materials. Schools often skip over this one in basic chemistry, though anyone working in industrial labs will spot it in catalogues. This amino acid takes center stage in the making of certain nylon plastics—specifically, as the key part for creating nylon-8. Nylon ropes, conveyor belts, and some types of synthetic fabric start with this chemical.
How the Textile World Leans on This Compound
Every time a new, durable synthetic material comes onto the market, you can bet industrial chemists have toyed with molecules like 8-aminooctanoic acid. Factory floors—busy with looms and presses—benefit from stronger fibers. The acid leads to nylon variations with a unique balance between flexibility and toughness. Walking in a raincoat that shrugs off water or driving a car built with seatbelts and airbags that do their job in a pinch: both rely on such reliable polymers.
Role in Biomedical Experiments and Healthcare
Laboratory science doesn’t stick to the old classics. Medical researchers have tested nylon-8 derivatives as scaffolds for tissue growth. The flexible nature of these polymers helps shape tiny bridges where living cells can grow, multiplying to repair wounds. In my own experience assisting with undergraduate research, we marveled as custom-made films seeded new skin cells, giving burn victims hope for better treatment.
Supporting Green Chemistry Efforts
A lot of industries are searching for cleaner alternatives to classic petroleum-based materials. Since this amino acid can be synthesized from renewable sources, its applications open a path to more sustainable plastics. Some companies explore routes from plant oils or engineered microbes, which promises a smaller environmental footprint. This shift not only benefits people breathing city air but also saves factory workers from some legacy health hazards.
Challenges in Application and Looking Ahead
Practical application isn’t always smooth sailing. Some manufacturers run into trouble with costs, as newer polymers take longer to reach the same economies of scale as nylon-6 or nylon-66. Production itself asks for careful handling, especially given the reactivity of the amino group. It reminds me of early days in a research job—spilled chemicals, wasted batches, and a scramble to tweak temperatures and pH to improve yield. Overcoming hurdles means more cooperation across industry, academia, and regulatory agencies. While recycling old plastics has its own challenges, investing in reuse loops for nylon-like materials can bring down overall waste.
What Can Actually Make a Difference
Policies that reward green sourcing, tax breaks for bio-based plastics, and public research funding for the chemistry of amino acid derivatives will give industry a nudge. Engineers and scientists benefit from mentorship programs that get them talking across different fields. Bringing people from textiles, pharmaceuticals, and environmental tech to the same planning table lets fresh ideas travel faster.
Real progress, in my experience, usually grows out of partnerships. The curiosity that drives people to experiment with unusual molecules like 8-aminooctanoic acid can lead directly to stronger products, safer treatments, and a cleaner world.