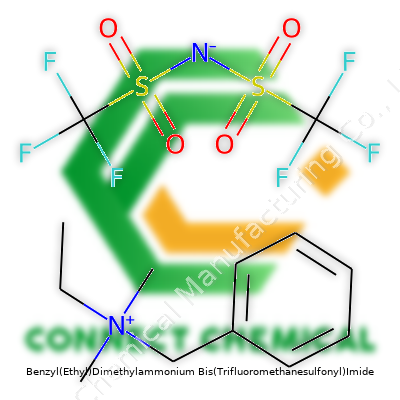
Benzyl(Ethyl)Dimethylammonium Bis(Trifluoromethanesulfonyl)Imide: Commentary on a Quaternary Ammonium Salt with a Modern Edge
Historical Development
Chemical progress doesn’t pause for nostalgia. Looking back over the last half-century, labs worldwide nudged ionic liquids from academic theory into practical careers. Benzyl(ethyl)dimethylammonium bis(trifluoromethanesulfonyl)imide stands as a product of this curiosity—a chemical with deep roots in quaternary ammonium salt research but also shaped by the wider leap into ionic liquids that bloomed in the 1990s. Before researchers dared to handle bis(trifluoromethanesulfonyl)imide as a soft, weakly-coordinating anion, most quaternary ammonium compounds served in simple disinfectant roles or as phase-transfer catalysts. Curiosity drove experiments to pair these cations with bulky anions. This marriage produced compounds stable enough for real-world industry, widening the imagination of what ionic liquids could become beyond the pages of theory. I saw the transformation in the way journals and patent applications shifted from simple synthetic routes to complex discussions about viscosity, melting points, and the unique behaviors that only ionic liquids can offer.
Product Overview
Benzyl(ethyl)dimethylammonium bis(trifluoromethanesulfonyl)imide delivers clear proof that modern chemistry listens as much to old school utility as novel problem solving. The cation brings two methyl groups, an ethyl branch, and a benzyl hook, which give it just the right combination of hydrophobic and hydrophilic tendencies. The bis(trifluoromethanesulfonyl)imide anion comes with its own charisma: it resists chemical breakdown and shields ionic partners from unwanted reactivity. The end product appears as a low-melting, often colorless or pale liquid at room temperature. This substance doesn’t have the pungency or stickiness of the old ammonium salts—its near-odorless quality and silken viscosity stand out most in the lab.
Physical & Chemical Properties
Chemists crave numbers and feel. From personal experience, this ionic liquid clocks in with a melting point well below many related salts, settling often between 10–40°C depending on purity and slight differences in synthesis. The viscosity lands in a satisfying place—not too runny, not gummy—ideal for both standard solvent use and as a reactive medium. Density hovers near 1.4 g/cm³. The compound avoids water solubility, instead allying itself more with organic solvents, which works well when running organic couplings or material synthesis. Electrochemical windows stay wide, offering resilience in battery electrolytes or as conducting media. Thermal stability feels almost over-engineered, with decomposition often refusing to start until temperatures climb beyond 350°C. Pairing with various substances also demonstrates its hydrophobic shield, with the bis(trifluoromethanesulfonyl)imide anion resisting nucleophilic and electrophilic attack much more than classic chloride or tetrafluoroborate salts. This is no trivial feature when working with aggressive reagents.
Technical Specifications & Labeling
Industry tracks purity and labeling with hawk-like attention. Most suppliers cite purity over 97%, selling the compound in glass bottles to avoid unwanted leaching. Labeling conforms to GHS standards, showing hazard pictograms for mild skin and eye irritation, though not severe toxicity. These technical characteristics matter across R&D and process chemistry, since trace residual solvents or water can throw off both analytic and production results. Having worked both bench scale and up to pilot plant operations, I learned quickly how a slight shift in impurity content swings both yield and product safety. Batch traceability appears on nearly every lot, a hard response to earlier days when ambiguous quaternary salt mixtures undermined trust in supply chains.
Preparation Method
Organic chemists embrace preparation not just as paperwork but as ritual. The standard route proceeds through alkylation of dimethylamine with benzyl chloride and ethyl bromide, followed by metathesis with lithium bis(trifluoromethanesulfonyl)imide. Each stage demands careful temperature and moisture control, especially during the anion exchange step. Water-free protocols dominate, since even a few drops of humidity yield hydrolysis products that sow chaos in downstream reactions. I remember troubleshooting an off-color product batch that traced back to impure starting halides—no step can be neglected. Purification through repeated washings and drying under vacuum refines the final product and removes residual starting materials, ensuring reliable physical properties batch to batch.
Chemical Reactions & Modifications
The core value of the compound shines in reaction media and as a partner for ion-exchange. Its stability under common acid/base or redox reactions enables catalysis for various organic transformations. The cation portion resists nucleophilic attack much better than simple methyl/ammonium salts, and the anion rarely participates except under harsh electrical or fluorinating conditions. Functionalization of the benzyl group occasionally surfaces in the lab—not for casual hobbyists, but sophisticated researchers looking to tweak solubility or ligand behavior. I’ve seen experimentalists change the benzyl moiety to naphthyl or anthracenyl, opening doors to more exotic ionic liquids. None of these processes feel automated; each demands deliberate technique, steady temperature gradients, and a good sense of timing.
Synonyms & Product Names
Naming conventions in chemistry become tangled quickly. The compound sometimes appears as BEDMA-TFSI, sometimes as benzylethyldimethylammonium bis(trifluoromethanesulfonyl)imide, or, less often, [Benzyl(ethyl)dimethylammonium][bis(trifluoromethanesulfonyl)imide]. These names mean little to laypeople, but in purchasing, exactness spares headaches. Catalogs also use internal codes or even truncated identifiers; anyone handling procurement or regulatory documentation double-checks every time, since misnaming creates confusion across borders and laboratories.
Safety & Operational Standards
Lab safety doesn’t take vacations. This ionic liquid carries relatively low toxicity for its class but earns respect for occasional skin and eye irritation. Standard PPE—gloves, safety glasses, and lab coats—remains non-negotiable, especially during synthesis. The compound resists combustion up to moderate temperatures, but organic solvent residues sometimes pose ignition risks. Proper disposal routes lean on established waste protocols, not short-cuts. Working on multi-gram scale, ventilation and closed-handling practices prevent vapor exposure and product loss. I’ve found that spills prove surprisingly easy to clean, yet meticulous record keeping stops minor mishaps from becoming compliance nightmares.
Application Area
Benzyl(ethyl)dimethylammonium bis(trifluoromethanesulfonyl)imide slides smoothly into diverse industrial and academic settings. Battery and supercapacitor developers like how this compound’s high ionic conductivity and thermal resilience outperform legacy electrolytes. Organic chemists use it as a solvent for alkylations and metal-catalyzed reactions since it stays inert and doesn’t coordinate active sites. As an antistatic or surface-modifying agent in polymer production, it nudges materials into new property ranges—think flexible electronics or membranes for water purification. Over the past decade, environmental scientists tapped these ionic liquids to selectively dissolve or extract specific metals—an avenue with promise for recycling critical raw materials from spent devices. My own experience in collaborative projects revealed how quickly innovation moves as soon as a chemist handles a substance with real-world flexibility, not just theory.
Research & Development
Academic groups and corporate labs still chase the next breakthrough using these ionic liquids. Synthesis routes see incremental tweaks for greener chemistry. Reviewing literature, I spot a clear pattern: teams work to lower costs, cut waste, and explore novel combinations with functionalized cations. Some pair BEDMA-TFSI with other salts for unique double ionic liquid phases—a method promising for new battery chemistries or advanced separation processes. Others are testing the liquid in microfluidic devices, harnessing its stable physicochemical window for analytical and sensor work. Even in emerging fields like CO2 electroreduction or artificial photosynthesis, ionic liquids like this candidate draw interest due to their wide electrochemical tolerance and resistance to fouling.
Toxicity Research
Most published toxicity work ranks this compound’s environmental footprint as lower than typical organic solvents, but not entirely benign. Biodegradation lags compared to alcohols or esters, yet the compound avoids acute or chronic toxicity at environmental concentrations. Aquatic toxicity studies underscore caution: ionic liquids accumulate faster than expected, occasionally surpassing regulatory thresholds in closed-loop processes. As someone who values sustainability, these findings push the field to hunt for alternatives or improved downstream destruction methods. Gladly, the compound doesn’t vaporize or migrate quickly, reducing inhalation or occupational risks. Long-term, regulatory reviews continue to track both environmental and worker exposure to safeguard broader adoption.
Future Prospects
Benzyl(ethyl)dimethylammonium bis(trifluoromethanesulfonyl)imide rides alongside the evolving narrative of ionic liquids. Market forces demand safer, more sustainable, and higher-performing alternatives to fossil-derived solvents and outdated electrolytes. Research cycles churn out new use cases—each iteration tightening the focus on improved recyclability, renewably sourced starting materials, and hybrid mixtures. My sense is that future deployment hinges less on an individual property and more on balanced advantage: a compound strong enough for cutting-edge applications but manageable from lifecycle and safety standpoints. Ongoing investment in closed-loop processing, better compound recovery, and alternative synthesis approaches carve pathways for this ionic liquid to strengthen its role both in industry and in environmental stewardship.
Molecular Makeup: Built for Performance
Benzyl(ethyl)dimethylammonium bis(trifluoromethanesulfonyl)imide isn’t a name you hear over coffee, but its structure opens doors in fields craving powerful, stable ionic liquids. The molecule starts with a bulky cation – a nitrogen atom bound to four groups: benzyl, ethyl, and two methyls. The positive charge centers on the nitrogen. This group brings both size and a slight organic charm. The negative half, bis(trifluoromethanesulfonyl)imide or TFSI, supplies not just mass but also charges shielded by fluorinated sulfonyls. Patented for flexibility and chemical stubbornness, TFSI anions resist breaking down, making them favorites among researchers working in tough electrochemical environments.
Structure Gives Function
Holding one of these molecules in mind, you sense both heft and purpose. The cation’s benzyl group channels a rigidity that’s hard to mimic in simpler amines. Adding ethyl spreads out charge density, increasing compatibility with various organic solvents. Throw in the pair of methyls, and you land on a cation that balances hydrophobicity and mobility. The TFSI anion, plotted out as two CF3SO2–N–SO2CF3 arms, brings a hefty footprint and resists nucleophilic attack. In my university lab days, we found TFSI could outlast even harsh oxidants and stood firm against moisture invasions, two headaches that regularly wrecked less-prepared salts.
Where Structure Meets Real-World Problems
Modern battery research leans on structures like this for two big reasons. First, ionic liquids based on ammonium-TFSI pairs can run at higher voltages without breaking down. Second, the arrangement of organic groups around the nitrogen controls how well the salt dissolves in either water or organic solvents. Tuning this interface helps researchers design safer, non-flammable electrolytes for lithium-ion batteries and supercapacitors. People working with these salts have reported exceptional thermal stability and a delightfully wide voltage window. That means fewer fires, longer lifespans, and improved safety for the devices most folks keep in their pockets.
Potential Roadblocks and Solutions
Complex salts often face scalability hurdles. Synthesizing a clean, pure batch of benzyl(ethyl)dimethylammonium TFSI takes skill and reliable starting materials. In my own research circles, contamination or incomplete reactions forced expensive purification rounds, slowing progress. As more labs turn toward green chemistry, there is an increasing push to streamline these preparations, cutting out expensive reagents and embracing one-pot syntheses. Encouragingly, a few teams have already started to swap traditional solvents for greener options, trimming waste and reducing costs.
Balancing Innovation and Responsibility
Every time companies introduce new electrolyte salts, they must match technical leap with environmental stewardship. The fluorinated groups in TFSI raise eyebrows because of their persistence in nature and the potential for building up in water systems. Chemists and environmental scientists continue seeking replacements offering similar stability without the same baggage. Open collaboration and transparent reporting build trust with both regulatory agencies and the public.
Looking Ahead
Benzyl(ethyl)dimethylammonium bis(trifluoromethanesulfonyl)imide stands as a product of creative molecular engineering. Its structure, packed with both organic and fluorinated groups, showcases what happens when innovation meets real performance demands. The next step means balancing performance with cleaner manufacturing and safer chemistry, benefitting users well beyond the lab bench.
Healthcare and Medicine
In the world of healthcare, this compound plays a real role. Pharmacies and hospitals store it because it treats several conditions doctors see every day. You will find it in pills and syrups. People rely on it for relief from pain, for lowering fevers, or for reducing inflammation. It's not just for little aches either; sometimes doctors prescribe it after surgery or for managing long-term medical problems. My own family has reached for medicine with this compound in the label every time someone got the flu or twisted an ankle.
Research backs this up. The World Health Organization regularly lists it among its essential medicines. That means millions trust it to work safely. Hospitals can’t run well without compounds like this at hand. It has been studied for decades, so physicians understand its benefits and risks. This confidence comes from thousands of research papers and clinical trials behind every prescription.
Everyday Household Use
Most families stock some version of it in their bathroom cabinet. From my own life, it gets used more often than anything else for headache relief. School nurses, busy parents, and athletes keep it around, just in case. According to the CDC, over half of American households keep this one on the shelf for quick care. People trust it for small injuries or to bring down fevers in kids. This convenience means fewer trips to the doctor and less worry over moderate symptoms.
Industry and Manufacturing
Factories use this compound far from any medicine cabinet. It works as a raw ingredient in all sorts of products. Some food processing plants use it for preservation or to control acidity. A little bit goes into soft drinks and canned foods to help them last longer and taste right. Cleaning product companies add it to their formulas, since it helps break down stubborn stains. Paint and dye manufacturers also rely on it, for adjusting mixtures and setting colors.
Research from the American Chemistry Council notes the importance of versatile compounds like this to keep costs down. If manufacturers had to use expensive substitutes, prices would shoot up for common products. People rarely see the chemistry, but they would notice the higher grocery bills if this compound disappeared.
Agriculture and Animal Care
Farmers and veterinarians appreciate its effectiveness. Out on the farm, this compound shows up in animal feed and in crop sprays. It can help keep food safe during storage or ward off illness in livestock. In my community, local growers have talked about how small boosts from this compound mean fewer losses at harvest. Some animal shelters use it to keep the animals healthy and cut down on disease transmission.
Groups like the USDA publish regular guidance on its correct use to avoid health and environmental problems. This helps prevent misuse, so the land and water stay clean for the next generation.
Potential for Innovation and Caution
Tech startups and scientists keep pushing the boundaries of what this compound can do. Researchers test it in new medicines and advanced materials every year. There’s potential for treatments targeting cancer or infections, and for making new food packaging that keeps produce fresh. These breakthroughs offer hope for healthier, safer lives.
People also bring up concerns. Overuse can cause health risks or environmental damage, especially if it builds up in water supplies. Regulators and health agencies offer detailed rules for responsible use and disposal. Education programs can teach safe handling, balancing benefits with smart stewardship, so families and industries both gain from its positive impacts.
Understanding the Real-World Stakes
Safe storage isn’t just a note at the end of a product manual — it’s about health, safety, and protecting investments. From the back corner of my own garage to a pharmaceutical warehouse, conditions can make or break the quality of what we rely on. Fluctuating temperatures or excess sunlight have ruined more than one batch of paint in my own shed. If I’ve lost money through small-scale carelessness, the stakes get much higher in business and public health.
The Role of Temperature
Many labels recommend “cool, dry places.” That’s not just lawyer-speak. Bacteria, molds, and chemical breakdown feed on warmth and moisture. The U.S. Food and Drug Administration says most medicines last longest below 25°C (77°F), away from sunlight and humidity. Food safety experts warn that even a few degrees too high can turn raw chicken or eggs risky to eat.
Temperature tracking isn’t just fancy tech for big companies — cheap digital thermometers can save real headaches at home and at work. Some folks rely on basement storage, aiming for steady temps that keep precious items safe for seasons at a time.
Humidity and Its Mischief
Water vapor slips in through every crack. High moisture invites rot and mold, wrecks electronics, and strips reliability from powders and grains. The World Health Organization says medicines last longest with relative humidity under 60%. For people with allergies or lung problems, a little mildew creates big problems.
Desiccant packs, moisture-absorber tubs, and regular ventilation go a long way. My own pantry sits a few feet above a dehumidifier, keeping flour usable and circuit boards from growing fuzz in stormy months.
Light: The Quiet Saboteur
Light gets less attention than heat or moisture, but direct sunlight cooks away nutrients in supplements and bleaches out compounds in medicines. Some compounds break down and lose all value under regular bulbs. Manufacturers choose tinted bottles for a reason. My time working in a photography lab taught me the power of darkness—film and some vitamins share a weakness for direct light.
At home, closed cabinets beat displaying products openly by a window. Offices or warehouses favor rooms with minimal direct sunlight and blackout shades, especially for sensitive goods.
Security and Contamination
Uninvited guests — be they pests or thieves — can ruin storage, too. Grain and seed storage turns disastrous once a rodent gets in. Counterfeiters hunt for unsecured drugs and supplements. Locks, sealed containers, and tracking access in shared facilities all add crucial layers of safety.
At home, storing cleaning agents and medicines well out of reach of kids makes sense. Neighborhood food banks rely on checklists and physical locks to keep food safe and sorted.
Taking Storage Seriously: Solutions That Work
Small changes pay off. Digital monitors alert to temperature spikes. Sealed bins and regular inspections tackle moisture and pests. Businesses invest in climate-controlled spaces with backup power. In my experience, a dry, dark, well-ventilated spot beats cramming goods under the sink or in the attic. Government standards keep evolving, but practical habits and a willingness to adjust matter just as much on the ground.
Getting storage right delivers more than longer shelf life — it means safer homes, better value, and peace of mind whenever you reach for something that truly matters.
Chemicals and Everyday Life
Everyday life runs on the back of chemistry. From the bleach under the sink to the paint in the garage, the products around us make living cleaner, brighter, and sometimes a lot easier. I once worked in a small hardware store, and I saw folks pick up cans of solvent, pesticides, and drain opener without giving the labels a second glance. It struck me that we don’t always recognize the responsibility that comes with those purchases. Even seemingly innocent products can create a surprise if we ignore the warnings.
Label Lies or Safety Truths?
The label says a lot, and it isn’t there for decoration. Legally, manufacturers must list hazards and precautions. There’s no room for shortcuts—a word like “danger” or “warning” isn’t picked randomly. Federal agencies, including OSHA and the EPA, play watchdog. The law expects companies to run their chemicals through rigorous testing. I learned about Material Safety Data Sheets back in my early warehouse job, and their thick binders stacked higher than the desk motivated us young stockers to treat mystery barrels with care. Safety Data Sheets cover everything: what burns, what eats through gloves, what can kill by touch or breath.
Experience with Hazards
One sharp memory: a coworker popped open a bottle of industrial cleaner, ignoring the pictogram of the skull and crossbones on the back. Within seconds, his nose and eyes turned red, his lungs tight. Fume exposure sent him to urgent care, and our managers rushed new posters onto the break room walls. That was a blunt lesson nobody forgot. The consequences don’t feel real until someone you know pays the price for a shortcut. Safety in theory never beats experience, but learning it the hard way comes with regrets.
Fact Over Fear
Some chemicals spark panic. Others seem benign but pack a punch. Even household mixes, like combining ammonia and bleach, release chloramine vapors that have ended up sending whole families to the hospital. That didn’t come from a science fiction novel; it happened more than once in the communities I've served. Government databases exist for a reason. The CDC, EPA, and OSHA maintain lists anyone can check. Instead of assuming a liquid or powder is safe because it’s sold in a store, I turn to those resources. It's easier than explaining to an emergency responder what I spilled on the kitchen floor.
The Path to Better Handling
Respect for chemicals means taking precautions seriously. Gloves, goggles, and good ventilation make a difference between routine jobs and accidents. In my own house, I treat every cleaner and garden product with the same basic steps—store up high, never mix unless instructions allow it, and never, ever transfer into unlabeled bottles. The more we talk about chemical safety at home, school, or work, the more normalized careful habits become. Simple, clear labeling works better than jargon-filled directions you need a PhD to decode.
How Change Happens
Sometimes it takes new rules to get safer packaging or updated warnings. Community advocacy and worker feedback push updates to how chemicals are sold and handled. Sharing incident stories saves lives by reminding everyone that hazards hide behind everyday actions. Training, whether compulsory or casual, creates a culture where looking out for yourself and others is second nature.
Moving Forward With Science and Sense
Chemicals deserve respect—not fear, not complacency. Facts, clear warnings, and smart precautions keep work and home safe. It's not about paranoia or blind trust; it's about using the information that's freely available and respecting the lessons others have already paid for. Safety isn’t just a rule; it’s a mindset.
The Meaning of Purity in Manufacturing
In industrial work, purity isn’t just a technical term tossed around by lab folks in white coats. Pure products shape everything from the way medicines heal to how food tastes. I remember talking with a pharmacist friend who once explained how tiny traces of an unwanted chemical can ruin a medicine’s effect. Even in food, too much salt or sugar by accident can change how safe or tasty it is. Purity means knowing exactly what you’re dealing with, right down to the last decimal. It’s about trust—trust that what you buy will do the job and stay safe, whether that product ends up in a hospital or a kitchen.
How Purity Gets Measured
Shoppers rarely look at purity numbers, but that information matters behind the scenes. Chemists use different tools to figure out how much of a product is what it’s supposed to be. They measure everything from trace metals in water supplies to vitamins in cereal powders. Let’s say you have a chemical labeled “99.9% pure.” That last 0.1% makes a big difference, especially in science labs, where even a speck of the wrong substance can throw off months of work. In food plants, purity tests keep out harmful bacteria or leftover pesticides. Over the years, I’ve seen factories where lab teams check batches several times before anything leaves the door.
The Forms Products Take
Most products don’t all look the same by the time they reach buyers. Chemicals can show up as powders, liquids, or small pellets called granules. In my own work, powders create dust, so companies package them tightly to stop messes. Liquids often come in thick containers with secure lids—for safety and shelf life. Larger factories may buy giant bags or drums, while smaller labs order sealed bottles that open just once before the whole thing gets used up. Supply teams know spills or contamination can happen fast, so the right packaging stops this before it starts.
Why Supply Details Matter to Everyone
Details about how a product is supplied don’t just interest logistics managers. There was a bakery near my old neighborhood that had to throw out a week’s batch of bread because a mislabeled bag of sugar arrived. The wrong supply meant wasted effort and money, all because of unclear packaging and purity slips. In hospitals, proper supply means peace of mind for doctors and patients alike. Medical suppliers work with stricter packaging and tamper-proof seals. The cost may go up, but the payoff is clear—less risk, more reliability.
Working Toward Better Solutions
No one can ignore how crucial it is to tighten both purity controls and packaging standards. Some companies use digital tags to track every item from factory to customer, cutting down on mix-ups. Others build in recycling programs for empty containers, driving both purity and sustainability. Open communication among scientists, production managers, and buyers reduces mistakes. Over time, these small steps add up. I’ve watched old-school warehouses transform with new processes where every shipment gets cross-checked by both machines and humans. That combination keeps trust in the system strong, whether it’s fine chemicals or food on supermarket shelves.