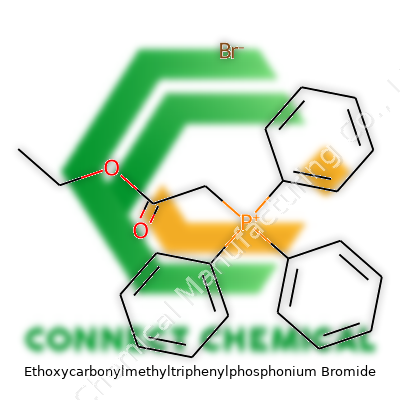
Ethoxycarbonylmethyltriphenylphosphonium Bromide: More Than a Reagent
Historical Development
Shifts in organic chemistry through the twentieth century created an appetite for new reagents. In the 1960s, the Wittig reaction gained a foothold, giving chemists the power to construct carbon-carbon double bonds with precision. Researchers needed stable phosphonium salts to push the field forward, and soon, lab benches filled up with cleverly designed phosphonium bromides. Among these, ethoxycarbonylmethyltriphenylphosphonium bromide stood out because of its ability to generate ylides with an ester group, not only expanding synthetic routes but also improving access to important molecules across pharmaceuticals and agricultural chemistry. Its history ties directly to a string of breakthroughs in carbonyl chemistry, and its introduction marked a reliability boost for chemists wanting specific products without messy byproducts or drawn-out purification.
Product Overview
Ethoxycarbonylmethyltriphenylphosphonium bromide serves as a stable, crystalline white salt. Its formula, C23H22BrO2P, tucks triphenylphosphine into a handy reaction handle, plus a bromide anion providing stability and solubility. In the lab, this salt quickly turns into the corresponding ylide when treated with base, letting it add ethoxycarbonyl groups across double bonds—a basic need in complex molecule building. Companies stock this reagent in research-grade and analytical-grade packaging for universities, startups, and big companies alike, as it solves problems in synthesis pipelines from bench-scale trials up to early industrial adoption.
Physical & Chemical Properties
As a solid at room temperature, ethoxycarbonylmethyltriphenylphosphonium bromide brings practical handling to organic synthesis. It looks snow-white and fine-textured, with a melting point typically falling near 190–195 °C, which helps in both transport and storage. This salt dissolves best in polar solvents such as methanol, ethanol, and dimethyl sulfoxide. Its chemical behavior is defined by the phosphonium center bridged by three phenyl rings and a methylene group capped with an ethoxycarbonyl function, giving it both stability and a knack for easy transformation into reactive intermediates. It resists unwanted side reactions until called upon, staying inert enough for months in properly sealed glass at room temperature.
Technical Specifications & Labeling
Manufacturers typically offer detailed specifications for this compound: chemical purity above 98%, verified by HPLC or NMR; moisture below 1% for predictable weight by mass; and assured single-component identity (CAS 3564-49-4). Accompanying labels display hazard pictograms, precaution codes, and batch-specific assay results. Alongside European and U.S. regulatory compliance, labeling stretches to include storage temperature (15–25°C), non-flammable classification, and directions for minimizing contact. QR codes increasingly feature, providing instant access to safety datasheets and applications, making it easier to trace sourcing from bench to reagent fridge.
Preparation Method
Chemical syntheses trace back to well-documented steps: triphenylphosphine reacts with ethyl bromoacetate in a polar aprotic solvent—often acetonitrile or dichloromethane. Heating this mixture jump-starts nucleophilic substitution, with the arylphosphine attacking the methylene carbon next to bromide, forming the salt and liberating triphenylphosphonium as the cation. After a few hours, cold filtration and repeated washing with diethyl ether help purify the crystalline salt from byproducts or unreacted starting material. Yield ranges from 80–90% with scaling adjustments, and the compound proves amenable to recrystallization in ethanol—a plus for achieving analytical grade batches on demand.
Chemical Reactions & Modifications
The magic of ethoxycarbonylmethyltriphenylphosphonium bromide comes out in the Wittig reaction, where it transforms into an ylide under treatment with mild bases, such as sodium hydride or potassium tert-butoxide. Ylides derived from this reagent add onto aldehydes or ketones, forging a carbon–carbon double bond and installing an ethoxycarbonyl group at the beta-position. Chemists exploring functional diversity find that subtle tuning of conditions—temperature, base, substrate—modifies outcome, sometimes even controlling stereochemistry of the resulting alkene. Outside its role in direct olefination, clever groups have designed follow-up reactions like elimination, Michael addition, and hydrolysis. As a reagent, it keeps providing starting points for peptides, pharmaceuticals, and even conjugated polymers, where the installed carbonyl group acts as a hook for later stages.
Synonyms & Product Names
Throughout literature, companies and research teams refer to this compound under several names. Ethoxycarbonylmethyltriphenylphosphonium bromide stands as the most systematic. You’ll also see names like (Ethoxycarbonylmethyl)triphenylphosphonium bromide, ECP-TPP bromide, or Triphenylphosphonium, (ethoxycarbonylmethyl)-, bromide. Catalogs from Sigma-Aldrich, Alfa Aesar, Tokyo Chemical Industry, and several other global suppliers include similar references. Indexing by CAS number (3564-49-4) helps avoid confusion, especially as synonyms crop up in patents, supplier sheets, and journal articles.
Safety & Operational Standards
Any lab routine with phosphonium salts calls for strict safety precautions. This compound irritates skin and eyes, and inhalation of dust brings respiratory discomfort. Handling guidelines suggest gloves, eye protection, and good ventilation. Spills get handled with non-sparking tools, followed by disposal in chemical waste. Storage aims for dry, cool, and tightly closed containers, clear of oxidizers or acids. Safety Data Sheets describe both acute and chronic effects. Research teams store salt bottles in chemically resistant secondary containment, meeting university or company guidelines. These routines aren’t just about checking boxes—they keep researchers healthy and prevent minor accidents from becoming much bigger issues.
Application Area
People draw on ethoxycarbonylmethyltriphenylphosphonium bromide for more than one synthetic trick. Its biggest home lies in complex molecule construction supporting drug discovery. It offers medicinal chemists a direct tool for adding carbon–carbon bonds to molecules loaded with sensitive groups. Process development chemists use it in scaled reactions for medium to large molecule production. Researchers in agricultural chemistry and materials science incorporate its reactivity for making crop protection agents or fine-tuning small polymer chains. Teaching laboratories rely on its reliable behavior for demonstrating the Wittig reaction to students. This reagent’s flexibility means it finds a foothold anywhere someone needs to transfer a carbonyl group into challenging molecular frameworks.
Research & Development
Innovation with ethoxycarbonylmethyltriphenylphosphonium bromide stays lively. Recent papers detail how reaction conditions affect yield, selectivity, and scale. Some groups work on immobilizing the salt on solid supports, making it easier to separate from product mixtures. Others have adjusted the ester group, swapping ethoxycarboxylate for other alkyl chains in hopes of expanding substrate scope. Collaborations across academia and pharma companies drive new methods for cleaner synthesis or asymmetric control. In my own undergraduate days, losing a batch of this salt mid-step stalled our project, nudging us to tweak conditions and streamline purification. These stories highlight how direct experience with this reagent shapes both technique and broader chemical intuition.
Toxicity Research
Studies of toxicity fill a persistent need for chemical responsibility. Standard animal testing points to moderate acute toxicity, though lasting effects remain limited under controlled use. Some gastrointestinal or respiratory irritation appears at higher concentrations, pushing for strict lab controls. Chronic effects receive sparse attention in open literature, but risk management focuses on exposure controls and prompt medical evaluation after accidental contact. Environmental persistence runs low, as hydrolysis and microbial breakdown clear the salt from most lab and production waste streams. For teams using kilograms per year, investing in containment, proper PPE, and user training stands as the first line of defense.
Future Prospects
Innovation keeps ethoxycarbonylmethyltriphenylphosphonium bromide visible at the front lines of organic chemistry. Improving access to chiral ylides through various substituent tweaks, streamlining purification, and scaling green manufacturing methods sits near the top of most company wishlists. Academic groups look for new reactivities, searching for ways to make the Wittig step even more versatile. Industry eyes automation, hoping for flow systems that recycle catalyst beds made from this class of reagents, reducing solvent waste and improving throughput. Teaching labs and research firms keep pressing for lower-cost, higher-purity stocks. The next few years could see variants with lower environmental impact or extended shelf stability, reflecting broader trends in sustainable synthesis.
Breaking Down the Name
Some compound names seem intimidating until you strip them down. Ethoxycarbonylmethyltriphenylphosphonium bromide falls into the "mouthful" category. The formula boils down to C24H24BrO2P. Looking past the complexity, there’s a structure packed with meaning for chemists and researchers alike.
Where Naming Meets Structure
Each part of the name reflects a piece of the molecule’s architecture. Those three phenyl rings—connected to a central phosphorus atom—aren’t there for show. That phosphorus holds a positive charge, making the cation important for how this compound acts in chemical reactions. On the other side, the ethoxycarbonylmethyl group tacks onto the phosphorus, bringing unique chemical opportunities. The bromide acts as a counterion, balancing out the charge, but it’s not just a tag-along; it influences solubility and reactivity.
Why This Matters for Researchers
During my graduate years, these kinds of phosphonium salts played a role in preparing ylides for the famed Wittig reaction, a staple for creating carbon-carbon double bonds. Every time someone took that bottle off the shelf, the formula mattered. Knowing exactly what atoms are present—C24H24BrO2P—ensured reproducible results. Chemists rely on these formulas to predict how a molecule will perform, react, or even decompose.
Synthesis projects often hinge on precision. Even a single atom’s omission or addition changes everything. I once ran a reaction with a batch an undergrad mixed, only to find yields lower than expected. Tracing it back, the problem lay in a mislabeled bottle; the formula didn’t match. That lesson sticks with me whenever I teach students—always double-check the molecular formula.
Beyond the Label: Practical Implications
Ethoxycarbonylmethyltriphenylphosphonium bromide isn’t just about building blocks or textbook reactions. Its precise formula lets researchers map out safety precautions. Elements like bromine point to potential hazards; it can irritate skin and requires proper handling. In some labs, phosphorus compounds need special disposal procedures. That level of care starts by knowing exactly what you’re dealing with, right down to the atoms.
Working Toward Safer and More Efficient Chemistry
Reliable formulas pave the way for safer lab work and reliable syntheses. Contamination and mislabeling still cause accidents and failed experiments. Some labs have started using barcodes and digital tracking systems for chemicals to cut down on these errors. Every improvement in tracking and identification promotes trust in results and better chemistry for everyone.
In my experience, transparency about chemical composition builds confidence among coworkers, encourages proper respect for hazards, and actually saves money by preventing costly mistakes. Sharing clear, accurate formulas helps not just newcomers, but also veterans retracing a path to a past discovery.
Moving Science Forward
Scientific progress leans on clear communication. The chemical formula C24H24BrO2P isn’t just a string of letters and numbers—it’s the foundation for safe, reproducible, and innovative research.
Packed with Power for Building Molecules
Organic synthesis relies on chemistry’s building blocks. Some chemicals show up again and again in labs because they help connect pieces—pieces that lead to new medicines, specialty materials, or simply a better way to design a molecule. Ethoxycarbonylmethyltriphenylphosphonium bromide stands out as one such specialist in this toolkit.
Forging Carbon-Carbon Bonds
Anyone who has spent time in the lab making new molecules learns that carbon-carbon bonds serve as the backbone for most organic structures. The Wittig reaction has earned a reputation for bringing two molecules together: one with a carbonyl group and one with a phosphonium ylide. Ethoxycarbonylmethyltriphenylphosphonium bromide serves as an ylide precursor. Once prepped with a base, it reacts with a carbonyl compound—often turning an aldehyde or ketone into an α,β-unsaturated ester.
Why reach for this tool? It gives chemists a way to control how pieces get stitched together. Predicting the outcome means fewer wasted materials and less time spent on troubleshooting. In the real world, that means researchers get to working prototypes, clinical candidates, or publishable results more quickly.
Impact on Drug Discovery
New therapies almost always start at the bench. Compounds containing α,β-unsaturated esters show up everywhere in drug candidates—from anti-inflammatory agents to antivirals. Ethoxycarbonylmethyltriphenylphosphonium bromide turns out to be one of the go-to reagents for introducing this group cleanly and efficiently.
Back when I worked alongside medicinal chemists, I saw this salt pop up on Scripps or Harvard chemical shelves. The idea was simple: reach a target compound in three steps instead of seven. Less hassle, less purification, better yields. That’s what moves projects past early research toward animal studies and, rarely, to something patients might try years down the road.
Making Complex Structures in Fewer Steps
Chemists love shortcuts that don’t meaningfully sacrifice quality. This reagent helps shorten the path to products with multiple double bonds, rings, or even odd-shaped natural products. New materials for electronics, light-absorbing dyes, and chemical sensors also grow out of these lab-made pieces.
The fact that both academic and industry chemists keep EWGs like the ethoxycarbonyl group in their toolbox speaks to trust built on real results. Published syntheses—from antitumor agents to plant protection products—show how this tool works outside a theoretical context.
Cleaner Methods and Sustainable Chemistry
Flipping through the latest literature, one sees a shift toward greener chemistry: less hazardous waste, lower energy, greater selectivity. Old-fashioned reagents sometimes create byproducts that are tough to isolate or clean up. Ethoxycarbonylmethyltriphenylphosphonium bromide shows up in protocols offering milder conditions. Instead of nasty solvents or harsh acids, researchers often manage reactions at room temperature or in water-compatible mixtures.
Cleaner chemistry means less environmental impact. This helps satisfy both regulatory bodies and an industry increasingly expected to justify its processes from safety and sustainability perspectives.
What Needs Attention
No chemical works in a vacuum. Availability, price, and purity always factor in. There’s also a push for more scalable, industrial-friendly versions of these transformations. People now talk openly about waste management—how to deal with side products like triphenylphosphine oxide, for example. More investment in recycling and recovery may pave the way for broader industrial uptake.
Final Thoughts
Chemists keep returning to ethoxycarbonylmethyltriphenylphosphonium bromide for a reason. It helps turn ideas into new molecules, ties directly to real-world needs like new medicines, and fits with the modern push for cleaner, more efficient chemistry. That’s the mark of a real workhorse in my lab and countless others.
Storing That Compound: More Than an Afterthought
Anybody who’s worked with chemicals, whether in a lab, warehouse, or even at home, eventually comes up against a universal truth: you can’t just toss a compound in any old spot and expect it to stay stable. Humidity, light, temperature—all these details shape what happens inside that container. People sometimes learn this the hard way. I once saw an old reagent degrade on a shelf near a heat vent, turning from white powder to a brown mess, just because it absorbed moisture from the air. That incident wasted both money and lab time and drove home how storage choices ripple through an entire operation.
Why Storage Mistakes Happen So Often
Storing compounds isn’t about just keeping them out of reach or putting a lid on a jar. Consider sodium metal. If someone leaves it exposed to air, it reacts violently with moisture and oxygen. Even common aspirin can break down if exposed to too much heat or humidity. Problems start when people skip the details on the label or the safety sheet. Sometimes, budget limits or lack of storage space push people to cut corners, stacking bottles wherever there’s room. These choices can turn simple compounds into hazards or reduce their effectiveness, leading to failed experiments or even safety incidents. For example, peroxides in solvents can build up and cause explosions if kept too long in warm conditions.
Understanding Temperature, Light, and Moisture
Most compounds prefer a stable, cool setting, out of direct sunlight. That’s why so many storage guidelines suggest temperatures around 20°C (68°F), unless the label says otherwise. Light-sensitive compounds suffer photodegradation, their bonds breaking down under even everyday fluorescent bulbs. For example, vitamin C solutions lose potency fast under bright light. Desiccators or sealable containers with drying agents help protect hydroscopic compounds, which grab water out of the air and clump together or even change chemically. Organic peroxides break down if kept warm, releasing gases that pressureize sealed bottles and sometimes rupture them.
Safety Practices That Just Make Sense
Every chemical has a Material Safety Data Sheet outlining storage needs. These details aren’t just suggestions—they’re risk barriers. I always double-check these sheets before shelving anything new. Mixing incompatible compounds can be disastrous; acids stored near bases risk creating toxic fumes or even fires. Storing strong oxidizers like potassium permanganate near organics is a recipe for disaster. Even storing mild chemicals alongside each other without separation sometimes leads to slow reactions, especially during summer heat waves.
Better Storage, Fewer Headaches
It doesn’t have to cost a fortune to improve storage. Dedicated chemical cabinets, segregated by hazard class, help avoid mix-ups. Clear labeling with dates prevents using degraded material that may have lost its punch—or turned hazardous. Regular audits and throwing out expired compounds keep the workplace safer. Investing in temperature monitors in storage areas tells you immediately if a fridge or freezer breaks down, letting you act fast. Training matters, too; I’ve seen new staff try to store peroxide-forming solvents near sunlight just because it seemed convenient. Sharing stories and proven practices goes further than any rulebook for keeping people and compounds safe and reliable.
Safe Storage Builds Trust in Results
Every test, every product, every experiment depends on knowing the compound in use acts the way it should. Careless storage chips away at confidence—sometimes in ways you don’t see until something goes wrong. Reliable temperature, proper containers, clear labeling, and a culture of recheckingwhat’s on the shelf all support better science and safer workplaces, plain and simple.
Chemistry Isn’t Just for the Lab
So, you've come across ethoxycarbonylmethyltriphenylphosphonium bromide. The name itself feels like a chemistry tongue-twister, but in reality, this compound shows up time and again in organic synthesis. It’s used a lot in making alkenes through the Wittig reaction, which anyone who’s been deep in synthetic organic chemistry will remember—hours at the bench, glassware stained with the purple tinge from triphenylphosphine byproducts, and the satisfaction of dry crystals at the end of purification. But before any reaction takes off, knowing the molar mass isn’t just a science-fair trivia—it’s the springboard for accurate measurement, solid yields, and reproducibility.
The Straight Numbers
Let’s skip the guessing games. The molecular formula here is C22H20BrO2P. Each atom in this formula carries its weight, so getting the molar mass means adding them up based on atomic masses. A little math:
- Carbon (C): 22 atoms × 12.01 g/mol = 264.22 g/mol
- Hydrogen (H): 20 atoms × 1.008 g/mol = 20.16 g/mol
- Bromine (Br): 1 atom × 79.904 g/mol = 79.904 g/mol
- Oxygen (O): 2 atoms × 16.00 g/mol = 32.00 g/mol
- Phosphorus (P): 1 atom × 30.97 g/mol = 30.97 g/mol
Add it up for a total molar mass right at 427.26 g/mol. That’s the number you want to write on every flask and bottle so your experiments don’t end up as guesswork.
Why Precision Matters
For those who have spent years hunched over a scale, weighing powdery solids that look awfully similar, accuracy goes hand in hand with trust—trust in results, trust in the data you submit, and trust in your own process. One misstep in recording or calculating molar mass, and you end up with byproducts, wasted reagents, or unexplained results that turn a ten-minute reaction into a week-long headache. If you’re running short on an expensive starting material or running a pilot batch for scale-up, wasted input due to sloppiness on the calculator stings more than just the wallet. Students and professionals alike learn quickly: never cut corners on the basics like molar mass.
Room for Errors and How to Dodge Them
Memory can be a trickster. Many times, I’ve watched a reaction fizzle or give strange TLC patterns because someone trusted an internet database without double-checking the numbers. It pays to tally atoms yourself and confirm with trusted resources like PubChem or the CRC Handbook. Printing out these numbers and taping them to your workspace beats flipping through conflicting web pages or deciphering old notes scribbled in haste. Sticking to this practice can mean the difference between smooth experiment flow and time lost troubleshooting a problem that could’ve been prevented.
Maintaining Good Scientific Habits
Staying organized means more than labeling bottles. It’s about building a foundation, one calculation at a time, where mistakes don’t balloon into disasters. The simple act of recording the molar mass—like 427.26 g/mol for ethoxycarbonylmethyltriphenylphosphonium bromide—gives future researchers, lab partners, or even your own forgetful self a clear place to start. Real science builds on this type of careful, everyday step. In fact, it’s these habits, combined with a healthy skepticism and thoroughness, that keep chemistry moving forward safely and reliably, batch after batch and year after year.
Why Bother Talking About Lab Chemicals at All?
Lab chemicals often get used without much thought, but ignoring the risks can bring real trouble. Ethoxycarbonylmethyltriphenylphosphonium bromide has a long name, and behind it sits a compound that’s seen in organic synthesis. Chemistry teachers share stories about how a moment’s carelessness with chemicals can mean skin burns, breathing issues, or worse. All those years in the lab, I learned nothing replaces respect for chemical risks, no matter how routine the work feels.
Hazards: Not Just Lab Math
This chemical features triphenylphosphonium—a family that often hints at toxicity for people and aquatic life. Many phosphonium compounds act as irritants. They mess with skin, eyes, or airways on contact. Even worse, long-term exposure, sometimes through poor handling, brings the chance of chronic health problems. Researchers have reported that highly charged organic salts, like this one, sometimes lodge in body tissues and throw off normal cell activity.
The ethoxycarbonylmethyl group adds another wrinkle: it doesn’t play nice with open wounds or mucous membranes. Labs keep Material Safety Data Sheets (MSDS), and the guidance is clear. For ethoxycarbonylmethyltriphenylphosphonium bromide, the sheet lists warning symbols for toxicity and environmental hazard. That’s not just paperwork—those red diamonds on the bottle are real signals.
Real-World Safety Practices
Every job with this chemical starts before even touching the bottle. Proper gloves—nitrile, not latex—block leaks through tiny holes. Goggles beat safety glasses for splash protection. Coats and sleeves stop dry particles or sticky solutions from hitting skin. Even hours spent hunched over a beaker change nothing: one moment without protection leads to a chemical burn that lasts weeks.
Fume hoods sidestep breathing risks. Small amounts of dust pack surprising power. I once watched a colleague work outside the hood for just five minutes—hours later, sneezing and a raw throat hit hard. A few micrograms of airborne compound, invisible and unnoticeable, do their damage silently.
Spills should never get the wipe-and-forget treatment. The right practice uses absorbent pads designed for chemical spills, and bags go straight to a sealed hazardous waste bin. Water alone only spreads the chemical. After handling, hands get scrubbed twice with mild soap, not just a quick rinse. Clothes get changed, since powders ride on cuffs and collars.
Storage and Training
Ethoxycarbonylmethyltriphenylphosphonium bromide stays in tightly sealed amber bottles, far from food and drink. Temperature changes and light can break down similar compounds, so a cool, dry storage area matters. Someone new in a lab needs proper training before working near these chemicals—just shadowing a senior coworker won’t do. Refresher training, even once a year, helps everyone notice problems before larger accidents happen.
Raising the Bar: Industry Responsibility
Companies and schools have a duty to mark bottles, teach workers, and post clear hazard signs. Clear labeling in plain English saves lives. Emergency showers and eyewash stations belong in every room. Routine drills keep the response muscle strong.
Safety slips never come from malice. People get busy, distracted, or tired. But with chemicals like ethoxycarbonylmethyltriphenylphosphonium bromide, one shortcut can cost real health. In the end, basic respect for chemistry and sticking to clear safety steps make the biggest difference between a routine workday and a trip to the ER.