M-Methylbenzyl Chloride: Insights, Challenges, and Future Prospects
Historical Development
The early days of organic synthesis saw chemists looking for ways to modify simple hydrocarbons to engineer more advanced molecules. M-Methylbenzyl Chloride, which features a methyl group attached to a benzyl chloride core at the meta position, popped up in scientific literature as synthetic chemists explored chlorinated aromatics. By the mid-20th century, demand for nuanced aromatic building blocks grew as the pharmaceutical and agrochemical sectors hit their stride. Researchers realized that introducing a methyl group onto the benzyl position influenced both reactivity and selectivity, especially for downstream syntheses. Today, it stands as a quiet workhorse in the toolkit of chemical manufacturers, with its use quietly stretching across multiple scientific and industrial disciplines.
Product Overview
M-Methylbenzyl Chloride doesn’t make headlines like some designer molecules, but its value becomes clear in the context of building more complex structures. The chemical structure—a benzene ring with a methyl group in the meta position, plus a reactive chloromethyl arm—gives it a unique footprint. This compound makes it easy for labs to introduce a functional handle onto the aromatic ring, helping connect pieces during multi-step organic syntheses. Many intermediates, including pharmaceuticals and flavors, rely on starting materials just like this. Its availability in both bulk and smaller laboratory scales keeps it accessible for scientists tackling both small-batch research and large-scale production.
Physical & Chemical Properties
This liquid typically presents as colorless to pale yellow with a pungent, sharp odor. Boiling point hovers around 205°C, with a melting range under -10°C, which signals a practical stability under most lab conditions. Solubility skews toward organic solvents, like ether and chloroform, steering clear of water. The molecular weight checks in at 140.6 g/mol, not especially heavy, but enough to influence handling practices. That reactive benzylic chloride group tends to drive substitution and elimination reactions, drawing nucleophiles easily. Stability wanes in alkaline conditions, and exposure to sunlight can spark unwanted side reactions. Labs need to respect both the volatility and reactivity—left open to air, the substance can emit corrosive fumes over time.
Technical Specifications & Labeling
Most suppliers adopt clear technical benchmarks for this chemical, often specifying purity at 98% or higher. Labels call out the CAS number along with hazard identifiers, since exposure risk looms large for skin, eyes, and respiratory tracts. Density averages 1.08 g/cm³, and documentation usually advises storage in tightly capped amber bottles, away from heat and moisture. Safety datasheets anchor every purchase, flagging both emergency and best-practice guidance. Consistent specifications help keep processes reproducible—any drift in purity or moisture content can muddy downstream reactions or leave impurities in an active ingredient. That makes traceability and batch data an everyday matter for practitioners.
Preparation Method
Making m-Methylbenzyl Chloride often starts with methylbenzene (toluene) derivatives. Chlorination usually unfolds under controlled conditions with reagents like thionyl chloride or phosphorus trichloride. Direct alkylation followed by chlorination sometimes crops up, especially if the target is the meta isomer. The process spits out hydrogen chloride gas, demanding robust ventilation and scrubbing gear; I’ve seen how careless handling can quickly become a nightmare in small labs. Reaction monitoring checks for over-chlorination, which leads to hard-to-remove byproducts, so real-time analysis isn’t just a luxury—it’s a necessity. Effective washing and purification finish the job, with distillation drawing out the desired product. Keeping solvents dry throughout is essential or else hydrolysis can sabotage overall yield.
Chemical Reactions & Modifications
Anyone looking to swap the chlorine for other functional groups finds fertile ground with this molecule. Nucleophilic substitution opens the door to amines, ethers, and even sulfide analogs, making it a conductor in orchestration of broader syntheses. In one project, I watched a team turn m-Methylbenzyl Chloride into a range of ethers for flavor chemistry applications. The aromatic ring itself also tolerates additional modifications, especially electrophilic aromatic substitutions. If push comes to shove and you need to build more exotic structures—a nitrile, an aldehyde, or even a quaternary ammonium compound—this starting material lends a ready hand. Polymer chemists even tap this compound for crafting specialty plastics, anchoring other building blocks onto aromatic frameworks.
Synonyms & Product Names
Depending on the supplier and end-user, m-Methylbenzyl Chloride answers to several names. You might see it sold as 3-Methylbenzyl Chloride, 1-(Chloromethyl)-3-methylbenzene, or meta-Methylbenzyl Chloride. Some catalogs drop the numerals and simply call it m-Toluyl Chloride, which can add to the confusion for less-experienced buyers. Each synonym reflects a local nomenclature tradition or historical context. Regulatory documents and customs codes hinge on these alternate product names, and mixing them up can trip up logistics or compliance paperwork. Clearing up ambiguities early in procurement or regulatory filings saves later headaches.
Safety & Operational Standards
Handling chlorinated benzylic compounds means one slip risks burns, breathing problems, or lingering contamination. M-Methylbenzyl Chloride carries GHS hazard statements for corrosivity and respiratory sensitization; goggles and gloves aren’t optional. In my own work, splash protection and proper chemical fume hoods made the difference between routine operations and chemical incidents. Emergency eyewash and showers belong nearby, and colleagues keep spill kits handy. Training for first-time users includes recognizing symptoms of exposure, since delayed effects sometimes mask the seriousness of an accident. Waste and off-gas runoff require scrubbing and special collection; you can’t pour this down the drain or allow it to evaporate indoors. Regulatory compliance doesn’t allow much wiggle room—local, national, and international rules set firm boundaries for use, storage, and disposal.
Application Area
In pharmaceuticals, m-Methylbenzyl Chloride paves the way for drug intermediates. Medicinal chemists lean on it to attach or modify pharmacophores, tailoring molecular structure to target key receptors. Agrochemical innovation taps it as a precursor for targeted herbicides and insecticides, where fine control over aromatic substitutions can mean the difference between crop safety and ecological risk. Flavor and fragrance houses use it quietly in the background, exploiting its substitution power to coax out new scents and tastes. In specialty polymers, its aromatic core anchors side chains for advanced resins or specialty plastics, enhancing product toughness or chemical resistance. Lab-scale synthesis benefits too: many research projects use this chemical as a foundational piece for piecing together new candidate molecules, often in university or contract research settings.
Research & Development
Current R&D efforts look at both greener synthesis routes and fine-tuning selectivity. The move toward less hazardous chlorination agents—think phase-transfer catalysts or less noxious chlorinating reagents—aims to cut down environmental impact. I’ve seen academic partnerships pursuing routes where catalytic amounts of halogenating agents trim waste and boost atom economy. On the analytics side, breakthroughs in real-time monitoring help catch off-spec product before it reaches the market. Structure-activity relationship studies help drug developers push the limits of what substituents can bring to downstream bioactivity. Open communication between academia, contract manufacturing, and industry accelerates both process optimization and diversification of application fields.
Toxicity Research
Chlorinated aromatics occupy a gray area in toxicology. Animal studies connect prolonged exposure to central nervous system effects and liver stress, with skin and mucous membrane irritation even at lower doses. Inhalation of vapors isn’t only a minor concern—workers exposed during transfers have reported both acute and chronic health issues. Regulatory agencies list exposure limits, and the chemical’s reactivity means accidental ingestion or mishandling can result in corrosive burns. Ongoing research explores the environmental breakdown of this compound, as incomplete disposal means potential for bioaccumulation or groundwater contamination. Labs and manufacturers now pair handling guidelines with environmental monitoring, mapping out fate and byproduct risks across the compound’s lifecycle.
Future Prospects
M-Methylbenzyl Chloride stands at an inflection point as green chemistry standards evolve and regulatory scrutiny tightens. The push for safer synthesis and improved waste management dovetails with industries demanding higher-quality inputs. Optimized preparation protocols and in-line analytics promise both cleaner product and less hazard for workers and the environment. The ongoing search for new applications, especially in pharmaceuticals and advanced materials, suggests a stable future—even as the compound’s handling and supply chain practices face new expectations. Collaboration between chemists, engineers, and safety experts can turn old challenges into new strengths, keeping this tried-and-true molecule relevant in tomorrow’s labs and factories.
Understanding M-Methylbenzyl Chloride
Plenty of chemicals float under the radar, yet play bigger roles in daily life than people recognize. M-Methylbenzyl chloride counts as a strong example. This chemical, an organic compound with the formula C8H9Cl, looks like just another clear liquid to most folks, but the work it does stretches far beyond a simple appearance.
The Roots: Sourcing and Basic Facts
Producers often start with toluene to make m-methylbenzyl chloride by adding chlorine in the right spot on the ring. This step matters mainly for industrial use, so chemists get a starting point for all sorts of specialty compounds.
Where Science Meets Real Life
Most people bump up against this compound through goods that sit on store shelves. Agrochemical makers rely on it as a building block to whip up pesticides and herbicides. The fight against crop pests rarely happens without a basket of effective molecules, and m-methylbenzyl chloride helps tick that box.
Pharmaceutical companies see m-methylbenzyl chloride as a valuable stop on the synthesis roadmap, too. They use it as an intermediate, meaning they turn it into something else that actually helps in treating illness. For example, certain antihistamines and even cancer drugs start their chemical journey passing through compounds made from m-methylbenzyl chloride.
Plastics and resins benefit as well. If you’ve come across sturdy coatings or specialty adhesives, chances are molecules built from m-methylbenzyl chloride helped improve durability or chemical resistance. It does not bring about those changes on its own, but serves as a connector in the chain.
The Safety Side: Risks and Precautions
Most folks will never handle m-methylbenzyl chloride directly, and that’s for the best. It irritates skin, eyes, and the respiratory tract, so industrial users wear serious protection and follow tough safety rules. Accidents with this compound can lead to long-term health issues, so no shortcuts belong in the process. In groups where workers know the risks and get real training, the threat of exposure drops sharply.
Real-World Impacts and Oversight
Governments do not let companies handle this chemical without close supervision. In countries with chemical manufacturing plants, agencies check for compliance, track transportation, and set disposal standards to cut down environmental damage. This kind of oversight matters because mishandling these types of chemicals can harm groundwater and air quality. As more people worry about clean water and air, keeping a strong eye on chemical life cycles makes sense from a public health angle.
Having worked inside a chemical plant, I remember each shipment’s paperwork and the feeling that no detail felt too small. Mistakes with these intermediates not only cost a company money; they put real people at risk down the line.
Better Paths Forward
With growth in green chemistry, researchers keep hunting for alternative base chemicals and safer reaction methods. If a new path looks promising—where plants make similar compounds or safer molecules do part of the job—companies dive in. The push for sustainability isn’t just good for public image. It can cut costs, sidestep regulatory headaches, and protect everyone who comes in contact with similar chemicals.
Progress can feel slow at times, but real momentum comes from solid science, real-world experience, and a willingness to try something new in an industry that often clings to the tried-and-true.
Why You Really Need to Pay Attention
I’ve worked with plenty of specialty chemicals, and M-Methylbenzyl Chloride (MMBC) stands out. Even seasoned lab workers sometimes underestimate what it can do if mishandled. MMBC causes chemical burns, releases toxic fumes, and harms lungs if inhaled. Industry data and safety boards both back this up. Prevention always works better than trying to fix burned skin or a chemical splash in your eye. This kind of risk does not just affect workers — it hits loved ones if clothing or tools carry the chemical home, or if improper storage poses danger long after the shift ends. Real safety means treating MMBC as seriously as it deserves.
Protective Equipment: No Corners Cut
Experienced lab managers know proper gear keeps people out of the hospital. With MMBC, splash goggles mean the difference between clear vision and scarring. Nitrile gloves block more than a quick tingle—they stop the chemical from soaking into skin. A sturdy lab coat, closed shoes, and a chemical-resistant apron add layers of insurance. If you expect vapor — running MMBC in a reaction or bottling it — go with a real respirator instead of a surgical mask. That’s not just lab policy; it’s solid advice from NIOSH and OSHA, groups that exist because too many people have been hurt by shortcuts.
Ventilation: Fresh Air Isn’t Just a Comfort
You notice straight away if a chemical lab runs a strong exhaust system. That hum means vapors vanish before they hit your nose or lungs. MMBC likes to form choking fumes that do damage before your body can say “enough.” A well-ventilated fume hood limits risks fast. Some skip hoods to save money, but I’ve watched people cough and tear up from mild exposure even with the hood running. Without one, those vapors hang in the room for hours, seeping into hair, clothes, and everyone’s lungs. Proper fans and hoods keep more people safe than any poster or lecture ever could.
The Value of Good Habits and Training
Labs shape their safety records by the little habits workers choose every day. Training newbies to treat MMBC with respect reduces “oops” moments that cause panic. In my experience, people who anticipate spills or splashes act before problems grow. Employees who understand why spills matter show stronger safety records. Posting spill kits in easy reach and making eyewash stations obvious can turn a bad accident into a minor cleanup. Supervisors who check labels and maintain routine drills spot mistakes early instead of trusting luck.
Emergency Steps That Actually Matter
Quick action trumps theory. If MMBC spills on skin, lots of running water—no hesitation. Contaminated clothes go straight off. Vapor exposure means hitting the fresh air, loudly signaling for help if lungs seize up. Large spills trigger alarms and lock down the area to save others from exposure. Those moves matter, because MMBC doesn’t wait for you to look up instructions. Hospitals see fewer severe cases from people who train for the worst.
Why Storage and Disposal Aren't Afterthoughts
Once a container leaves your hand, you trust storage to keep it safe until next use. Keeping MMBC cool and dry reduces pressure build-up or leaks. Securing lids stops fumes. Storing away from water and oxidizers avoids violent reactions. For disposal, following state and federal hazardous waste rules may feel like paperwork, but dodging those guidelines can lead to fires, environmental fines, or physical harm. Companies that take disposal seriously rarely show up in accident reports.
Understanding M-Methylbenzyl Chloride
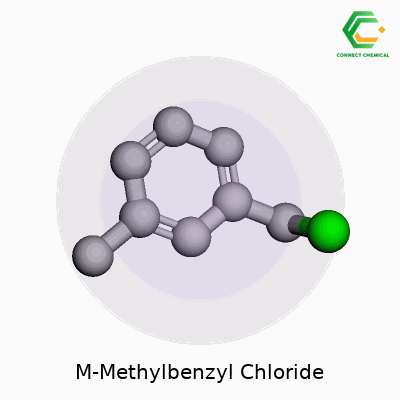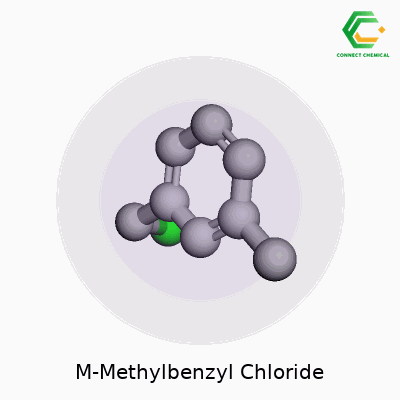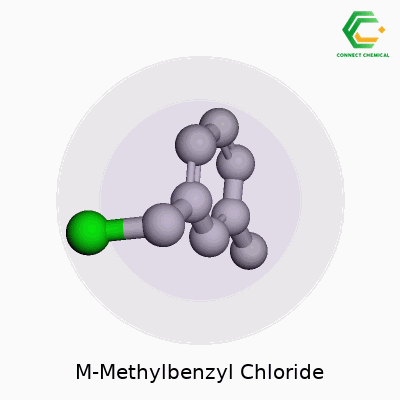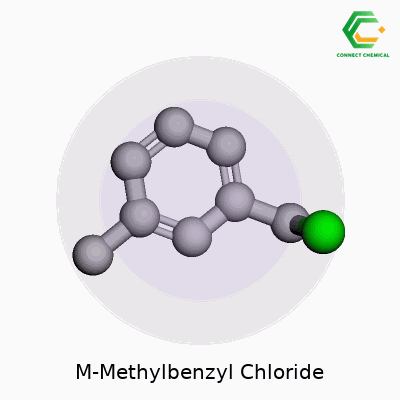
M-Methylbenzyl chloride carries the chemical formula C8H9Cl. To picture this molecule, think about a benzene ring — a classic six-carbon circle from high school chemistry — with a methyl group (a CH3 group) attached at the meta position relative to a benzyl chloride group. If you number the ring starting with the chloromethyl (–CH2Cl) at position 1, the methyl group ends up at the 3-position.
Molecular Structure at a Glance
The molecular structure is not especially complicated, but these small tweaks around a benzene ring change the way the compound acts, both chemically and biologically. The structure starts with benzene as its backbone, known for stability and the strong resonance of its π-electrons. Add a CH2Cl group, and then swap in a CH3 group at the meta location. This seemingly tiny adjustment leads to a unique profile.
Exposure to benzyl chlorides in the lab brings caution and respect. I once worked on a project synthesizing organic intermediates, and we always had to double check the label, since changing that methyl group from meta to ortho or para led to very different properties — melting points, reactivity, and even toxicity.
Where Accuracy Matters
M-Methylbenzyl chloride finds its way into the production of specialty chemicals, often showing up in pharmaceutical research or as a starting point for flavors and fragrances. Its reactivity comes from the benzylic chloride group, which makes it a strong electrophile. That means it acts like a chemical “magnet” for nucleophiles, ready to form new bonds — something synthetic chemists count on when building complex molecules.
Mistaking its structure for an isomer’s can cause a mess, both in the lab and in safety handling. Para or ortho analogues behave quite differently, reinforcing the practical value of clear, trustworthy chemical notation.
Potential Hazards and Responsible Use
Like most chlorinated benzylic compounds, M-Methylbenzyl chloride brings health risks through inhalation or skin contact. Research from the National Institute for Occupational Safety and Health (NIOSH) highlights the toxic and potentially carcinogenic effects of benzyl chlorides. Proper engineering controls, like vented fume hoods and gloves, become non-negotiable. Many companies mandate SDS consultation before opening a single bottle. From my years in chemical manufacturing, I’ve seen how small oversights with chlorinated aromatics can lead to significant incidents, so repeating safe handling is worth the extra effort each time.
Industry Reliance and Possible Alternatives
M-Methylbenzyl chloride’s usefulness lies in its ability to add specific structures to target molecules, streamlining synthesis and saving weeks of bench time. Yet chemists and manufacturers continue to look for safer, greener routes. Approaches based on direct selective methylation or less toxic halogenating agents draw increasing attention, although achieving the same efficiency is still a challenge.
Every compound, even one as simple-sounding as M-Methylbenzyl chloride, serves as a reminder to weigh both utility and safety. Working with this molecule means staying up on current literature and government guidelines — a principle that helps chemists create responsibly and move the field forward.
Understanding the Risks
M-Methylbenzyl Chloride doesn't just sound intimidating. This clear, colorless liquid packs a punch—it's flammable, reactive, and highly irritating to the skin, eyes, and lungs. Years working around chemicals taught me to treat every bottle of this stuff with a level of respect you usually reserve for a king cobra. Many overlook just how quickly a little carelessness can lead to injuries, fires, or ruined equipment.
Why the Right Conditions Matter
Walk into a well-run lab, and you'll spot a few essentials: proper ventilation, clean storage areas, and a keen eye for labeling. M-Methylbenzyl Chloride stays stable only if it’s kept dry and cool. Humidity can trigger hydrolysis, releasing acidic fumes you definitely don’t want in your face. Temperatures above room level speed up decomposition or, worse, make the vapor spread even faster. Keeping this chemical happy means storing it in a tightly sealed container, away from sunlight and heat sources. I once saw an old glass bottle left near a window; after a few sweltering afternoons, the cork gave out and filled the lab with pungent fumes. Since then, climate control and minimal light exposure have been my gospel.
Containers and Compatibility
Use glass or high-quality polyethylene containers, and check for even the smallest cracks or worn seals. Metal containers react with the compound and bring a whole new set of hazards. Labels on these containers should stick well and spell out the full chemical name, plus hazard warnings. No room for shorthand or faded stickers. That practice saved us more than once—new team members can spot trouble in an instant, even when they’ve never handled the substance before.
Separate from Incompatibles
M-Methylbenzyl Chloride should live far from oxidizers, alkalis, acids, and moisture. Store it on its own shelf in a chemical-resistant cabinet, never stacked above other reactive agents. Organizing chemicals for quick identification and separation prevents a world of accidents. A colleague once mixed residues by mistake because someone stacked incompatible reagents together; the minor spill that followed could’ve turned out much, much worse. Segregation may feel like a hassle late in the day, but it saves more than time.
Ventilation and Emergency Gear
Even tiny leaks fill a room with that sharp, choking odor before you realize it. A well-ventilated storage area—preferably a fume hood or cabinet with exhaust—is non-negotiable. I learned early that the quality of the ventilation setup often spells the difference between a safe workplace and a potential ER visit. Alongside each cabinet, set up an eye wash station and an emergency shower. The best glove for the job: nitrile, not latex. Keep spill kits within reach and routinely let folks practice quick clean-up drills.
Reliable Inventory and Training
Take stocking seriously. Use a robust log—digital or old-school clipboard. Track quantities and expiration dates. Encourage everyone, from trainees to bosses, to sign off after each use. This habit guards against forgotten open bottles and identifies vanishing stock right away. Comprehensive training beats any poster or memo. Every person needs to handle M-Methylbenzyl Chloride with focus and confidence, which only comes from hands-on instruction and clear, consistent reminders.
Final Thoughts on Responsibility
Every chemical mishap I’ve seen usually boils down to sloppy routines or overconfidence. M-Methylbenzyl Chloride won’t grant anyone a second chance. Respecting storage guidelines is about protecting people’s lives, not just ticking boxes on a safety audit. Solid preparation transforms risk into routine—never the other way around.
Living Close to Chemical Risks
Many of us never think twice about what happens inside chemical factories, but chemicals like m-methylbenzyl chloride create risks that extend well beyond the lab. This compound often gets used in making dyes, pharmaceuticals, and agricultural chemicals. Just a whiff or a splash can send someone to the emergency room. From personal observation working around industrial zones, folks tend to overlook clear risks until they're right in front of you.
What Makes M-Methylbenzyl Chloride Dangerous
M-methylbenzyl chloride comes with plenty of warning signs: its fumes sting the nose, and the liquid burns skin. Anyone who’s spent time handling industrial chemicals knows that this is not the kind of stuff to take lightly. Even a small spill can irritate eyes, burn skin, or trigger coughing fits. Studies have shown that long-term exposure may harm the liver and central nervous system, raising red flags for technicians who don’t have proper safety training.
Inhaling vapor can bring on headaches, dizziness, and nausea, and if things get bad, breathing can grow difficult. The worst cases documented in chemical accidents have included chemical pneumonia and long-term lung damage. And here’s a stark reminder: even healthy people struggle to bounce back from exposure if emergency action isn’t quick.
First Aid—React Fast, Think Smart
Sitting through safety training as an entry-level worker, the “golden minutes” message stuck—I learned that quick response often makes the difference. Splash to the eye? Run to the nearest eyewash station and rinse with water for at least fifteen minutes. Chemical burns on the skin require stripping contaminated clothing and hitting the safety shower for a long rinse. Anything less just increases the chance of permanent injury.
Breathing in m-methylbenzyl chloride fumes sends you straight into fresh air—don’t linger trying to tough it out. Sometimes, getting outside isn’t enough and oxygen support becomes necessary. If someone has trouble breathing, not waiting for a supervisor’s okay could save their life. Swallowing this chemical means not waiting for symptoms either—medical teams should get called immediately.
Why Preparation Saves Lives
Walking through older plants, it’s easy to spot where corners get cut—eyewash stations hidden behind stacked crates, safety showers blocked by pallets. Real life doesn’t pause for the perfect moment, so preparation is everything. Clear emergency access and routine drills save time, and that time saves tissue, eyesight, or breath. OSHA highlights the need for personal protective equipment (PPE): goggles, chemical-resistant gloves, and fitted respirators actually make a difference. I’ve seen coworkers avoid serious injury by respecting these simple rules, and I’ve also seen the opposite.
Knowledge alone doesn’t help unless people trust their training and equipment. Regular checks of safety gear, open conversations about near-misses, and commitment from every level of staff work better than paperwork and posted memos. Supervisors have a responsibility to do more than hand out warnings—they need to build a culture that expects danger, not denies it. That sense of watchfulness can mean the difference between a warning label and a tragedy.
Better Practices Mean Fewer Accidents
M-methylbenzyl chloride reminds all of us who spend any time around chemicals that there’s no substitute for respect and readiness. Industry standards call for well-marked safety zones, robust PPE, and clear response strategies for every shift. Lives rely on those details, so cutting corners for the sake of convenience isn’t just unwise—it’s reckless. Training, preparation, and communication remain the backbone of workplace safety. The science proves that small lapses quickly add up, especially around chemicals with hazards as clear as m-methylbenzyl chloride.