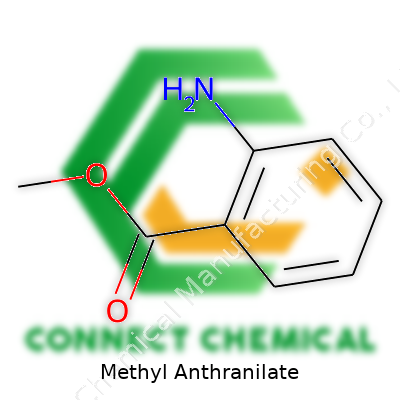
Methyl Anthranilate: Insights, Uses, and Industry Standards
Historical Development
Chemists have been fascinated by methyl anthranilate for well over a century. Back in the late 1800s, European analysts noticed its unique grape-like scent as a natural essence in certain flowers, particularly in orange blossoms and Concord grapes. Eventually, scientists isolated this ester and mapped out its chemical structure. Over decades, demand ballooned right along with the rise of the flavor, fragrance, and pharmaceutical sectors. Interest really spiked after manufacturers explored ways to synthesize this compound efficiently, since nature doesn’t offer it in the volume industry needs. The journey from flowers and grapes to laboratory synthesis put methyl anthranilate on the global map, feeding the need for vibrant, recognizable flavors in candies and sodas as well as in fine fragrances and specialty chemicals.
Product Overview
Methyl anthranilate is an organic compound often recognized by its fruity and floral aroma, closely associated with grapes. It turns up in both natural and synthetic forms. While many consumers unknowingly encounter it in everyday products like chewing gum, fruit drinks, and perfumes, those working within flavor or fragrance labs know it as a staple ingredient. Food technologists value its reliable performance and stability in formulations, while perfume designers prize it for its versatility, blending well with both sweet and floral notes. Its accessibility through chemical synthesis has also helped regulate costs and avoid over-reliance on botanical sources.
Physical & Chemical Properties
This ester shows up in laboratories as a colorless to pale yellow liquid. It generally has a boiling point hovering around 256°C and a melting point close to -24°C, with a distinctive, powerful aromatic profile. It dissolves best in alcohol and organic solvents, but struggles in water. Its strong scent threshold ensures that only a small concentration goes a long way when crafting flavors or scents. Methyl anthranilate’s density and refractive index make it relatively easy to identify and handle compared to other aromatic esters.
Technical Specifications & Labeling
Reliable suppliers offer technical sheets detailing purity, commonly topping 98%. Trace impurities must be tightly controlled, especially if the ingredient will be used in food or beverage applications. For regulatory compliance, the product label should carry not only the chemical’s name but also synonyms, batch numbers, and relevant hazard statements as dictated by GHS standards. Proper labeling reduces risks for workers and ensures traceability for recalls. Food industry guidelines, such as those set down by FEMA and Codex Alimentarius, also shape labeling and standards of identity for methyl anthranilate.
Preparation Method
Early extraction focused on isolating methyl anthranilate from natural sources using steam distillation. With advances in organic chemistry, the main route shifted toward chemical synthesis. The most common process involves the esterification of anthranilic acid with methanol in the presence of a dehydrating agent like sulfuric acid. Consistently high yield and purity rest heavily on controlling reaction conditions, so temperature, time, and molar ratios get closely monitored. Some manufacturers explore green chemistry alternatives to cut down on hazardous waste or switch to renewable raw materials, but synthetic production remains the dominant method.
Chemical Reactions & Modifications
This molecule participates in typical ester reactions—hydrolysis, for example, will revert it to anthranilic acid and methanol under acidic or basic conditions. Derivatives emerge through selective alkylation or acylation, which can tailor volatility or aroma characteristics. Chemists can manipulate its ring structure or swap methyl for other alkyl groups, producing close analogs suitable for new scents or flavors. Methyl anthranilate also reacts with oxidizing agents, which requires careful handling and storage, especially in large-scale operations where material loss hits bottom lines.
Synonyms & Product Names
In the trade, methyl anthranilate appears under various names. Industry catalogs often list it as “MA,” “methyl 2-aminobenzoate,” or “anthranilic acid methyl ester.” Within perfumery and food flavor houses, it might simply carry the label “grape ester.” Its widespread usage across continents and languages has led to a tangled web of synonyms, so chemical identifiers like CAS numbers play an essential role in avoiding confusion. These multiple names underline its importance across sectors, but also remind users to confirm specifications before use.
Safety & Operational Standards
While methyl anthranilate enjoys a history of relatively low toxicity, it still demands respect in a manufacturing environment. Unprotected exposure to concentrated vapors can irritate eyes or respiratory passages. GHS protocols require safety eyewear and gloves for handlers, plus proper ventilation for spaces where the compound gets processed or stored. Food and fragrance regulations limit permissible concentrations, especially since ingestion or skin contact in high amounts can cause unwanted side effects. Facilities handling large volumes incorporate spill control and rigorous documentation to assure both worker safety and product integrity.
Application Area
Flavor chemistry puts methyl anthranilate front and center for recreating grape, orange blossom, and other fruity flavors. Soda makers, confectioners, ice cream producers, and even pet food manufacturers rely on this compound. In perfumery, it adds lift to floral and citrus notes, showing up both in mass-market fragrances and niche blends. It has a lesser-known niche in agricultural chemistry, acting as a bird repellent in certain crop sprays—its strong flavor deters birds without major risk to the environment or consumers. Regulators and major brands alike keep an eye on usage rates to balance impact, consumer expectations, and safety.
Research & Development
Current research trends focus on uncovering more sustainable production methods. Some scientists study enzyme-catalyzed routes or fermentation using genetically engineered microbes, aiming for cleaner, greener processes. There’s work on analog development, too, as flavor and fragrance houses look for novel sensory experiences or more robust performance in challenging applications. Analytical chemists press for even lower detection limits in finished products, navigating evolving food safety standards. Ongoing partnerships between academia, industry labs, and regulatory agencies help flag any emerging safety concerns.
Toxicity Research
Historically, methyl anthranilate has tested with low acute toxicity in animal studies, although direct exposure in high doses can cause irritation or sensitization. Long-term studies have found no strong evidence of carcinogenicity, mutagenicity, or reproductive toxicity at levels present in consumer products. Food safety authorities like the FDA and EFSA regularly review new toxicological data and update allowable daily intake values, keeping public health risks in check. Research groups still screen metabolite profiles in humans and animals, refining risk assessments as analytical technology advances.
Future Prospects
Growing consumer focus on natural and traceable ingredients may push suppliers to revisit plant-based extraction, especially if crop yields improve or biotech breakthroughs offset high costs. Environmental regulations shape future production choices, pressing for cleaner methods and fewer hazardous byproducts. Innovations in synthetic biology might enable fermentation-derived methyl anthranilate with lower energy investment and less environmental burden. Developers in the flavor, fragrance, and agricultural sectors keep watch on legislation, consumer expectations, and supply chain issues, knowing that any advances in stability, safety, or sustainability will likely set the pace for tomorrow’s offerings.
Nature’s Aroma, Bottled
Methyl anthranilate might sound like a complicated chemical reserved for lab coats and textbooks, but it quietly shapes daily experiences. Walking through a grocery store, nabbing a grape soda, or picking up an air freshener for the kitchen, this compound plays a role. Methyl anthranilate occurs naturally in concord grapes, some citrus fruits, and a handful of flowers. The sharp, sweet grape scent hits the nose before a taste even lands on the tongue.
Food, Flavor, and the Illusion of Fruit
Any kid who has tasted grape-flavored candy likely recalls the intense, almost over-the-top perfume. That signature comes from methyl anthranilate. The food industry uses it in a range of synthetic grape flavors, weaving it into soft drinks, candies, and chewing gum. I’ve seen people surprised to discover their grape soda never spent time near a vineyard. This ingredient brings bold, familiar notes into recipes without relying on pricey or seasonal fruit.
The compound isn’t just for sweet treats. Sometimes it turns up in fruit-flavored yogurts or medicines, and it covers up the bitterness of vitamins or supplements. It’s one thing to swallow a chalky tablet, a whole other story to pop a grape-flavored gummy. Many parents probably owe a chunk of their success wrangling kids into taking medicine to clever flavor chemistry.
In the Mix: Fragrance and Beyond
Perfume makers reach for methyl anthranilate because it adds a distinct floral sweetness. Invitations to summer parties remind me of the scent hanging in the air—think citrus-laden perfumes or hair care products with that whisper of grape and orange. The fragrance industry leans on this molecule for cost and creative reasons. It helps them move away from rare natural oils, designer exclusivity, and sky-high prices.
Methyl anthranilate proves flexible, working its way into candles, soaps, and even industrial cleaners looking to leave a pleasant impression long after the job’s done. Sometimes a familiar smell takes the edge off harsh chemicals or gives a hospital room a touch of life.
Unexpected Role: Nature’s Pigeon Control
Cities use methyl anthranilate to keep birds at bay. Sprayed over sports fields or rooftops, it bothers the birds’ senses, steering them elsewhere. This knack for repelling pigeons and starlings relies on its safe profile for humans—people hardly notice while birds can’t stand it. Unlike harsher pesticides, this approach avoids injury and focuses on gentle deterrence.
Making Sure It Stays Safe
With methyl anthranilate popping up in food, fragrances, and pest control, safety matters. The US Food and Drug Administration considers it safe at common food use levels. Personal care and home products benefit from that broad review, but manufacturers still check for allergic reactions and sensitivities. I’ve met people with scent allergies who scan ingredient lists even on cleaning supplies, so clear labelling and transparency go a long way.
A Few Simple Solutions
People want products that bring the flavor or scent they expect without health risks. Testing and transparency aren’t just buzzwords; they build trust. Pushing for plant-based alternatives and lowering unnecessary additives means better options for all. Regulations and marketplace transparency help anyone understand exactly what’s in their soda or shampoo. If you love the grape flavor or pass on scented candles, it’s good to know what’s behind that choice.
Understanding Methyl Anthranilate
People find methyl anthranilate in the scent of Concord grapes and some flowers. Perfume makers value it for its sweet smell. Food companies use it to give candy and sodas a distinct grape flavor. Vineyards apply it for an entirely different reason: keeping birds away from fruit. It has a broad reach, and that means safety should always stay in the conversation.
Human Safety Profile
The FDA approves methyl anthranilate as a flavoring agent. This comes after toxicologists studied its behavior in bodies and the amounts people might eat or inhale. Many foods—including gum, drinks, desserts—have carried traces of methyl anthranilate for decades without clear links to harm. Still, nobody should let their guard down around chemicals, even those with a long record of use.
No evidence suggests it causes cancer, birth defects, or chronic health problems at the concentrations used in consumer goods. If someone works in a flavor factory or perfumery and breathes in large clouds of it every day, eye and throat irritation can happen. That kind of intense, workplace-only exposure just doesn't compare to the tiny drop in a grape soda.
Animal Exposure and Risks
Bird-repellent products with methyl anthranilate often get sprayed in orchards or vineyards. I’ve talked to local farmers in my region who try to keep starlings off their blueberries. They worry about safety for their own families, their pets, and the local wildlife. Agencies like the EPA require proof before approving these sprays. Studies with dogs, rodents, and other small animals show no lasting health damage at normal use levels.
For birds, this compound acts like a bad-tasting coating on fruit skin. They take a bite, hate the taste, and remember to avoid it. It doesn’t poison them—the science backs this up with repeated studies on pigeons, sparrows, and blackbirds. Domestic animals—like dogs and cats—tend to stay away from treated areas. Fish and aquatic life can face problems if too much of this runs off into ponds or streams; that's where accidents or misuse can become a problem.
Environmental and Practical Concerns
Used thoughtfully, methyl anthranilate delivers what vineyards and berry farms want: less ruined fruit without guns, nets, or lethal toxins. Still, applying any substance in bulk out in nature deserves a second look. Runoff, overspray, and buildup near ponds can harm local fish and frog populations. I’ve seen conservationists and growers hold workshops to mark out "no spray" buffer zones. These steps protect well water and sensitive habitats. Clear labeling and worker training prevent most accidental exposures.
Recommendations for Use and Oversight
Don’t just trust the "natural grape smell" label. Read about the product before heavy use. At home, go easy on scented sprays, even if they sound harmless. Farms should follow EPA-reviewed instructions and leave untreated strips as wildlife-safe corridors. Governments, researchers, and advocacy groups need to keep pushing for better studies and smarter application methods. Taking regular air, water, and crop samples helps catch surprises early.
It’s tempting to look for a simple answer—safe or not safe. The real world rarely works that way. Methyl anthranilate shows a high level of safety in food, fragrance, and carefully managed crop use, but common sense and vigilance should stay close at hand.
Why Farmers and Cities Use Bird Repellents
Birds eat more than we realize: sweet fruits, budding vegetables, corn, and even packaged food stored outside. In cities and on farms, flocks bring more than just a few lost crops or droppings. Cleaning bills add up, and orchard owners lose income. It makes sense to explore every tool that promises safer harvests and fewer pigeons perched above downtown benches.
What is Methyl Anthranilate?
Methyl anthranilate has been around for decades, found naturally in grapes and gardenias. It smells a bit like Concord grape juice, but its real appeal to manufacturers is how birds react. Science explains that birds sense this compound through trigeminal nerves in their beaks. They don’t taste flavor the same way we do, so scent-based ingredients make more sense than bitter sprays. As someone who spent a summer working in a blueberry patch, I remember loud flocks swooping in, ignoring shiny tape but fleeing after a new product (which later turned out to use methyl anthranilate) was sprayed.
Does Methyl Anthranilate Work?
Independent studies do point to results. According to research by the USDA, both red-winged blackbirds and starlings avoid food treated with methyl anthranilate. Controlled field tests saw reduced fruit loss on treated vines and bushes compared to untreated ones. Not every species reacts the same way. Sparrows, for example, often stay persistent even when their cousins steer clear. Someone who expects an invisible wall to keep birds out might feel disappointed.
The compound does not harm birds or people, and environmental groups have called it safer than older chemical repellents. Otters, raccoons, cats, and people don’t seem to mind the smell, so it targets just who you need it to.
The Real-World Challenge
No product solves everything. Heavy rain tends to wash away a spray, so application can get expensive by midsummer. Farmers sometimes spot residue on fruit and worry about taste shift, but grape-flavored blueberry isn’t actually a thing. The biggest downside for growers comes in timing and reapplication. Frequent reapplication for long seasons means more cost and labor. I’ve seen neighbors pool resources to buy large tanks and coordinate spray schedules because one patch left untreated becomes a magnet, ruining the group effort.
Smart Use and Next Steps
The Environmental Protection Agency considers methyl anthranilate safe, and most states allow its use on food crops. Many cities have started using it in parks and public squares, mixing it into sprays that coat statues and benches without leaving stains or slippery spots. Still, relying only on this compound never works out. Netting, noise deterrents, and habitat modifications produce stronger results together. New tech even looks at combining repellents with heat maps or motion tracking to target sprays only where needed.
The next move? Stay flexible and gather local results. Combining scientific reports with old-fashioned observation—checking if fewer birds pick at crops after applications—leads people to better solutions each season. Products like methyl anthranilate show real promise but require steady hands and a well-chosen plan to give the best shot at keeping flocks away without harming anyone involved.
Tracing the Origins
Methyl anthranilate pops up in all sorts of places. Ever bite into Concord grapes? That loud, tangy aroma owes a lot to this compound. Methyl anthranilate shows up in other berries, some flowers, and a few citrus fruits. It’s not just a lab thing — it’s a real part of nature, made by plants as a byproduct of how they defend themselves or attract pollinators. In nature, it’s not tucked away in giant vats, but you’ll find it in just enough quantity for birds and bugs to notice.
Food scientists and chemists figured out how to make this compound in factories a long time ago. There's a good reason: real fruit and flower extracts are expensive to harvest, and you don’t get a lot for your trouble. By making methyl anthranilate through chemical synthesis, industries such as food, fragrance, and pest control can scale up without emptying natural resources. The molecule from grapes and the molecule born in the lab are the same – it’s the journey, not the destination, that differs.
Personal Encounters: The Grape Candy Effect
Growing up, I loved grape bubble gum. That punchy, sweet scent is straight out of a methyl anthranilate bottle. Decades ago, the food world shifted mostly to the synthetic version. That’s what made the aroma so dependable. The taste you get from grape soda is reliable precisely because of synthesis, not a patchwork of harvested grape skins.
I remember visiting a vineyard at the peak of harvest; the sweet cloud in the air stuck with me. Later, learning the same aroma could be recreated in a lab felt a bit odd, but in reality, it’s a kind of practical magic. This flavor lets kids who never see a vineyard taste something close to the real thing.
Safety and Labeling Questions
Plenty of folks get nervous about the “synthetic” label. Here’s the piece that matters: the human body can’t tell the difference between methyl anthranilate made by a grapevine and what’s made by a chemist, so long as both are pure. Safety research backs up its use in food in small amounts. Science takes the mystery out of the molecule, focusing on dose and purity.
Where it gets tricky is in labeling. “Natural flavor” regulations in the US and Europe can confuse consumers. If the source is a plant or animal, you get a “natural” label, but synthesis usually gets tucked under “artificial flavor.” There’s room for clearer laws. People deserve real information, not marketing lingo — especially those with allergies or strict dietary needs.
Environmental and Economic Pressure
Extracting methyl anthranilate from fruit puts stress on the environment. Harvesting wild botanicals can affect local ecosystems and drive up cost, making the synthetic route an environmental fallback. On the other hand, sustainable technology, like fermentation with engineered microbes, opens new doors. Some biotech firms already use yeast to create flavor compounds, bridging the gap between “natural” and “synthetic.”
Moving Forward Together
The food and fragrance industries constantly face the tug-of-war between integrity and cost. As a consumer, I vote for transparency and honest science. There’s value in knowing where flavors come from, how they’re produced, and what impact they have on health or the planet. Greater transparency builds trust—taste and ethics can share the same table.
The Unexpected Reach of a Grape-Like Scent
Methyl anthranilate sounds like a lab experiment, but for most people, its sweet, fruity scent triggers memories. You stand under a grape arbor in late summer—the smell of ripe fruit fills the air. That aroma hits the nose because methyl anthranilate lives in grapes and a handful of other plants. Oddly enough, that same compound turns up in all sorts of places you might not expect.
In Food and Drinks, Taste Is Everything
Soft drinks, candies, and chewing gum producers turn to methyl anthranilate for its punchy “grape” flavor. The food industry keeps their trick simple: this molecule delivers a flavor people recognize instantly as Concord grape. Growing up, I could spot grape-flavored soda from across the basketball court just by the aroma. What I didn’t know—almost none of that soda came from actual grapes. Food scientists add this compound directly to drinks, cough syrups, lollipops, even breakfast cereals. Synthetic production kept the price down, which let companies flavor huge batches at once.
Even processed cheeses and baked goods carry a trace of this note. Its high safety rating from food regulators gave flavor houses a green light for inclusion. The U.S. Food and Drug Administration allows food-grade methyl anthranilate, so long as companies stay within certain tolerance levels. By the time it hits your taste buds, the concentration sits far below anything known to hurt people.
Fragrance Goes Beyond Flowers
Perfume makers chase all sorts of fruit and flower notes. Methyl anthranilate appeared on their radar more than a century ago. Classic niche colognes like the 1912 edition of Guerlain’s Après L’Ondée relied on it for a soft violet-lilac note. Even basic air fresheners rely on this compound to lift and soften harsh chemical smells in rooms and cars. Anyone who used a “grape” body mist in middle school carried this molecule around all day without knowing it. Private-label cosmetic producers keep using it since it blends easily and holds up well in formulation, unlike some essential oils that fade quickly.
The Odd Role: Bird Repellent
I learned about methyl anthranilate’s unsung talent in college, standing in an orchard with a crop scientist. They sprayed grape-flavored "bird repellent." Birds pick up the taste and steer clear of fields, especially in blueberries, cherries, and even on golf courses where geese turn into a nuisance. The science behind it? Birds have different receptors than mammals: they pick up the bitterness and dislike it. Years of controlled studies confirmed safety for people, pets, and livestock at levels used outdoors. The Environmental Protection Agency approved it as a bio-repellent, not a pesticide, so you don’t get the residue concerns or regulatory headaches that dog harsher chemicals.
Insect-Repelling Potential
Recent studies tracked methyl anthranilate as a mosquito deterrent. Early research found that mosquitoes avoid it, just like birds do. Formulation specialists keep tinkering with sprays and patches, hoping for something that keeps bugs at bay without toxic risks. This avenue looks promising, but companies still have hurdles to clear, especially with long-term exposure data and unpredictable results in wild conditions. Good science takes time, but the prospects look a lot safer than some older-generation insecticides.
What Actually Matters Here
Long-term, society benefits from compounds like methyl anthranilate—familiar, well studied, low toxicity. Responsible regulation, routine safety reviews, and keeping the public informed matter as much as any clever product. Consumers deserve real transparency about what sits in food, perfume, lotions, and garden sprays. Open access to safety information, honest labels, and a real conversation with regulators keeps trust intact. It’s worth remembering where familiar flavors and scents begin. Often, it’s a molecule like this—natural, adaptable, quietly woven through hundreds of everyday items.