Methylcyclohexenone: A Comprehensive Commentary
Historical Development
Back in the early 20th century, organic chemists started tinkering with cyclic ketones, hunting for new building blocks to drive flavor, fragrance, and pharmaceutical discoveries. Methylcyclohexenone found its first bit of fame as researchers worked out the best ways to tweak simple cyclohexanone rings and load them with distinct substituents. Wartime chemistry pushed the pace. Demand for new intermediates and practical scents spurred competitive labs in the US and Europe to patent routes and stretch the available cyclohexene backbone. Labs dug up new catalysts and reaction paths to get more methylcyclohexenone per barrel of starting product, cementing its place in both academic and manufacturing settings over the next fifty years. Seeing old journals with yellowed pages full of methylcyclohexenone experiments reminds me how knowledge advances—inch by patient inch, trial by productive error.
Product Overview
Methylcyclohexenone lands on the desk as a classic intermediate. It blends both practicality and versatility, straddling the chemical world with a six-membered ring and a single double bond. Its core role is to anchor synthetic strategies for everything from sweet-smelling perfumes to essential drugs. Fitting a methyl group on a cyclohexenone makes the structure nimble enough for a range of follow-up reactions, but sturdy enough to stand up to storage and shipping. No one ingredient sits alone on a glass shelf; methylcyclohexenone walks straight into formulations for fine fragrances and flavors, barely registering as the crucial step it often is. I’ve seen small companies run bench runs to nail down the right impurity profile, and big manufacturers optimize for purity, yield, and cost—proof that a simple structure can keep unlocking new chapters in applied chemistry.
Physical & Chemical Properties
Methylcyclohexenone shows up in the lab as a clear liquid, with a faint floral or nutty note, depending on which methyl and double bond position isomer touches your nose. Boiling points land around the mid-200s Celsius, so the compound tolerates gentle heating. Its moderate polarity keeps it soluble in common organics, while water barely mixes without help. The molecule has a carbonyl group that chews up electrons and makes it an eager partner for addition and condensation reactions. Anyone who’s worked with it knows the solution’s color can darken if you leave it under air; handling under inert gas holds back slow oxidation. These traits make the compound a reliable participant in both careful batch productions and quick exploratory reactions at the fume hood.
Technical Specifications & Labelling
Labeling usually tracks the usual suspects: methylcyclohexenone’s CAS number, batch date, purity percentage, and maximum allowed impurities such as water or residual solvents. Major suppliers display flash point data, density (measured between 0.93–0.96 g/ml), and NMR analysis peaks if requested. Color often falls below 10 Hazen units for fragrance work. Labs expect suppliers to vouch for trace metal content, as any iron or copper traces can speed up unwanted side reactions. Regulatory compliance certificates often ride along with each batch, making it easier for downstream users to tick off local product stewardship boxes.
Preparation Method
Synthesis pathways for methylcyclohexenone usually start from cyclohexanone or cyclohexene. The most relied-on route mixes cyclohexanone with methylating agents, typically through alkylation under basic or acidic conditions. Fine-tuning pressure, temperature, and catalyst choice shifts yields and affects how many side products form. Certain catalytic hydrogenations can pluck double bonds into just the right location. Experienced chemists keep a steady eye on reaction reflux to prevent over-reduction and maintain the proper ketone structure. Crude product often needs vacuum distillation or column packing to reach high enough purity for sensitive downstream use.
Chemical Reactions & Modifications
One strength of methylcyclohexenone: it keeps doors open for creative modification. That lone double bond and the reactive carbonyl invite a string of options for Michael additions, aldol condensations, and selective reductions. I’ve seen process chemists turn methylcyclohexenone into building blocks for pheromone mimics, using simple base catalysis. Grignard reactions snap onto the ketone carbon, spinning the nucleus into more complex rings or alcohols. Adding strong acids triggers cyclization, sending the molecule down new synthetic forks. Large-scale production lines often recycle their mother liquors and distillation cuts to squeeze out extra product, leaning on the compound’s ready reactivity to recover value.
Synonyms & Product Names
Over the decades, methylcyclohexenone picked up a handful of names, depending on which chemist or supplier you ask. You’ll still see 3-methylcyclohex-2-en-1-one or 4-methylcyclohex-3-en-1-one pop up interchangeably in catalogs and technical sheets. Trade names show up more often in the flavors and fragrances sector, where niche isomers carry specific codes to signal subtle variations in aroma. Understanding which isomer sits in front of you makes a massive difference in downstream reaction planning, especially if regulatory data tags a certain structure due to environmental persistence.
Safety & Operational Standards
Lab safety slips start to pile up when folks underestimate the pungency or toxicity of medium-weight organics, and methylcyclohexenone is no exception. Its vapor can irritate eyes and upper airways, especially above room temperature. Spills feel greasy under glove but evaporate slowly compared to familiar solvents. Material safety data sheets mandate goggles, impervious gloves, and strong exhaust. Waste streams funnel through tight-sealing containers, avoiding drains to stop small-scale bioconcentration downstream. The chemical’s reactivity means storage away from strong acids, bases, and oxidizing agents; small drums often live inside ventilated lockers. Effective training goes past rules—watching an experienced tech react quickly to spills or vapor leaks leaves more of an impression than a single sheet of do’s and don’ts.
Application Area
Demand across fine chemical, pharmaceutical, and fragrance sectors gives methylcyclohexenone a secure if unglamorous home. In perfumery, this molecule threads through many floral, nut, and woody notes, giving backbones to everything from luxury scents to everyday cleaning agents. Drug discovery pulled methylcyclohexenone into libraries of synthetic intermediates; medicinal chemists attach molecular fragments and couple it with amines or aryl groups, chasing better activity and off-target profiles. Agrochemical chemists keep this compound close for herbicide and pesticide synthesis, where minor tweaks in structure lead to changes in field performance. Even polymer chemists have dabbled with derivatives, using them as stabilizers or crosslinkers in specialty plastics. Anyone skimming patent filings from the last two decades will recognize how often this backbone pops up as a keystone step in routes to more complicated molecules.
Research & Development
Research into the versatility and safety profile of methylcyclohexenone really ramped up as green chemistry approaches took over university and industrial pipelines. Recent years brought a wave of efforts to trim waste and switch to renewable feedstocks. Catalysis teams look for better ways to run low-temperature methylation or biocatalytic cycles, hoping to lower energy demand and solvent bills. Analytical chemists keep developing detection methods, chasing down 1-ppm traces in complex consumer product matrices. Platform startups sometimes promise “drop-in” natural synthesis, but established players fight hard to meet the regulatory documentation requirements that have stiffened up since the 2010s. My own time in pilot plants showed that even a proven intermediate like this stays in the R&D crosshairs, as techs chase higher selectivity and lower environmental footprints, even for mature products.
Toxicity Research
Many cyclohexanone derivatives hover in a gray zone for toxicity. Lab animal studies on methylcyclohexenone suggest moderate acute toxicity through inhalation or ingestion, with repeated exposure leading to nervous system symptoms in some cases. Regulatory assessments lump it with “irritant” categories, but the complexity of real-world consumer exposure drives more rigorous studies, including in vitro toxicity screening. Newer data sets have started fleshing out metabolic breakdown pathways in mammals, flagging potential reactive metabolites. Environmental scientists keep an eye on aquatic toxicity, since the compound’s low water solubility and ability to stick in sediment may threaten small waterborne organisms if disposal isn’t controlled. Decades of small spills and process losses from the fragrance and pharmaceutical sector have already built up trace background levels in some industrialized waterways. Cross-checking animal data with long-term worker health studies remains patchy, so updated occupational exposure monitoring stays critical.
Future Prospects
Chemical building blocks with solid track records don’t often make headlines, but methylcyclohexenone’s flexibility means it won’t fade any time soon. As biobased production scales up and synthetic biology outfits climb into the mainstream, enzymes that build cyclohexenone rings from sugar feedstocks might drive the next wave of “natural” labels. Downstream, the compound’s potential for new derivatives in taste, scent, and targeted therapy markets gives it staying power. Tighter environmental regulations will push makers to share more real-world fate studies and run fresh toxicity screens, while consumers keep asking for “cleaner” materials in everyday goods. My view—shaped by years among R&D and operations teams—is that methylcyclohexenone won’t just survive these shifts. Instead, the story will grow, shaped by traditional chemistry, emerging biotechnology, and a lively debate over risk, reward, and responsibility in how we build the molecules that fill our shelves and shape our lives.
The Appeal of Unseen Ingredients
Not everyone has heard the word methylcyclohexenone, but many have come across products that rely on this compound. Its real-world presence goes beyond textbooks and labs. Methylcyclohexenone pops up most often in the world of fragrances, carrying scents with an efficiency that companies value. For those in the perfume trade, this compound delivers subtle earthy tones that stick around longer than many natural extracts. The persistence of that freshness or that “clean” sensation from a spray on laundry or skin traces back to the resilience of methylcyclohexenone’s chemistry.
What It Does in Fragrance Creation
Growing up in a household where cologne sat on the bathroom shelf, I watched adults debate the difference between synthetic and natural aromas. Many leaned on tradition, but science edged its way in. Methylcyclohexenone offers perfumers a way to extend a fragrance’s life. Synthetics like this one bring consistency. They help companies manage costs, which means more folks can afford products without sacrificing that polished scent. Instead of a short burst of freshness, these scents linger through meals, work meetings, and Saturday outings.
Hidden Roles in Common Products
Beyond perfume, this compound finds a seat in household cleaning products. Cleaners and air fresheners benefit from its ability to mask less desirable odors and maintain a clean atmosphere indoors. I’ve noticed its lasting effects on my clothes after laundry day and in that recognizable crispness that hovers after a room gets sprayed down. Methylcyclohexenone helps make sure the smell means clean, not just a short cover-up.
Why Safety and Quality Control Matter
Chemicals in household goods often spark questions about safety. Trust in a product depends on more than its smell or staying power. The fragrance industry remains tightly regulated—groups such as IFRA and the FDA set standards to lower risks. Manufacturers weigh risks, balancing innovation and safety. Families deserve transparency about what goes into their air and onto their skin. Proper labeling and honest ingredient lists offer peace of mind. That’s one step toward trust—a promise I want to see honored in every home.
Looking Toward Greener Alternatives
Conversations around synthetic ingredients circle back to sustainability. With people growing more health- and environment-conscious, the industry looks for options that reduce waste and environmental load. Investing in research for more biodegradable, less toxic substitutes could reduce methylcyclohexenone’s foothold, but progress takes time. Change involves partnerships across science, policy, and the marketplace. My own experience as a consumer has taught me to read labels and ask questions. The more we push for transparency, the closer industry gets to better solutions.
Empowering Consumers Through Information
Knowledge brings power to the conversation. Understanding what methylcyclohexenone does can help people choose what touches their lives each day. Companies that open the door to questions and explain their processes can earn deeper loyalty. Each step toward clear communication supports safer, smarter choices for families, workers, and anyone wanting to breathe in a little more safely at home.
Understanding the Risks
Methylcyclohexenone shows up in a handful of industrial processes. Anyone who works in labs or factories knows that even a quick whiff of chemical fumes can ruin your day, and some substances stick around longer than others. This one, in particular, can irritate the nose, eyes, and lungs. Some studies hint at possible liver or nervous system issues from repeated exposure. It’s tempting to think a little ventilation or occasional gloves will cover you—but history is full of folks cutting corners and paying for it later. Protecting yourself means staying aware of more than just the label on the drum.
Proper Ventilation and Controls
Work areas need a solid flow of clean air. Open windows and fans rarely cut it for chemical vapors. Proper exhaust hoods pull fumes away from the breathing zone—experience shows that even a small spill can affect the entire room. Relying on luck or nose alone rarely works. Regular checks of air filters and vent fans prevent buildup. In places where this substance gets used heavily, air quality monitors help catch leaks or high concentrations before someone gets sick.
Personal Protective Equipment
Splashing or breathing in this stuff creates problems. Labs and plants always post reminders to wear gloves, safety goggles, and lab coats. Nitrile gloves handle most chemical splashes better than latex. Good safety goggles and face shields keep drops and vapors out of your eyes. I’ve watched people skip these steps when in a hurry, only to regret it later—nobody likes the eye wash station at the end of a shift. A proper respirator beats a paper mask when fumes get too thick. Fit and maintenance of gear matter just as much as wearing it.
Safe Storage and Handling
Buckets or jugs holding methylcyclohexenone belong in chemical cabinets designed for flammable liquids—preferably behind locked doors, away from sunlight or heat. Clear warning signs cut down on mistakes. Pouring or transferring should happen over trays to catch spills. Extra absorbent pads and cleanup kits by the door come in handy the moment something goes wrong. I’ve seen more than a few spills caught in time because someone planned ahead. Don’t store this solvent near acids or bases—reactions might surprise you, and not in a good way.
Spill and Emergency Response
Even with the tightest protocols, accidents slip through. Quick response keeps people and property safe. Spills call for immediate evacuation if vapor clouds fill the room, and those on cleanup duty need proper gloves, goggles, and sometimes respirators. Closing the area and using absorbent pads or neutralizers speeds up recovery. Reporting all incidents allows a team to track patterns and improve safety down the line—not blaming people but preventing future mishaps.
Training and Building a Culture of Safety
Everyone who works with chemicals should know exactly what they’re handling. New hires and seasoned staff both benefit from refreshers and drills. Sharing stories—good and bad—about what can go wrong helps drive the message home. Leadership sets the tone when it comes to following safety rules; shortcuts make danger less visible but just as real. A workplace that values open communication and watches out for each other works safer and smarter.
A Closer Look at Methylcyclohexenone
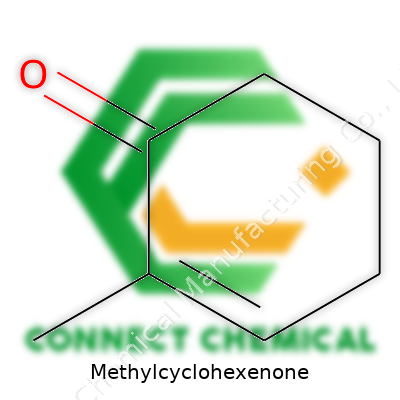
Methylcyclohexenone doesn’t get splashed across headlines, but it crosses paths with plenty of essential science and industry conversations. The molecule falls into the category of cyclohexenones, specifically shaped by a six-membered ring that’s not just closed, but also holds a double bond and two substitutions—one methyl group and one ketone group on the ring. Its formula, C7H10O, speaks to those elements: seven carbons, ten hydrogens, one oxygen.
Why the Details of Structure Matter
Understanding the skeleton of methylcyclohexenone means picturing a cyclohexene ring (six carbons in a ring, one double bond in the ring), a methyl group (–CH3) branching off, and a ketone (C=O) sitting in the ring. Chemically, the most common arrangement has the ketone at position 1, the double bond between carbons 2 and 3, and the methyl group at the 4-position. So, 4-methyl-2-cyclohexen-1-one best describes the version found in many labs and manufacturing plants.
Chemists care about every corner where atoms land because these differences shift the way molecules interact. In real terms, the presence of a double bond right near the ketone sets up the molecule for a range of synthetic reactions—think of it as giving a builder lots of strong anchor points. That methyl group doesn’t just sit quietly, either. It shapes boiling point, solubility, and how the smell spreads—traits that can turn a generic component into a key ingredient in flavors, fragrances, or chemical pathways.
Touchpoints in Real Life
In day-to-day experience, most people associate molecules like methylcyclohexenone with scents, flavors, or ingredients that disappear behind a brand name. Synthetic chemistry leans on this compound to open doors to larger molecular frameworks. During my undergraduate research, cyclohexenones like this one found their way into every other flask—a sign of both versatility and reliability. I remember spending afternoons trying to nail down reaction conditions, always hunting for that sweet spot where the ring stayed intact but new elements joined in just the right way.
In the health and safety arena, the chemical structure points the way to proper handling. The double bond and the ketone both signal reactivity that makes careful storage and controlled environments a must. Fact sheets and safety protocols look at that formula and translate it into gloves, ventilation, and awareness—basic moves that keep labs and factories running without headlines of mishaps or mystery odors.
Room for Improvement
Not every cyclohexenone compound needs to raise eyebrows, but industry gets a boost from paying close attention to little structural quirks. Investors, manufacturers, and product designers could all get more value by pressing for better data on how methyl group location tunes reactivity and performance. Academic collaboration stands as one clear way forward—lab results in the public domain help everyone from small businesses to regulators grasp what’s likely to happen if a new application hits the market.
More effort aimed at green chemistry could build on the structure, too. By choosing catalysts and conditions that respect the particular mix of double bond and ketone reactivity, greener processes might cut waste and energy bills. Students and researchers who trade ideas openly are often the first to spot these opportunities, turning what feels like an ordinary ring into a smarter, safer foundation for tomorrow’s products.
Understanding What You’re Dealing With
Methylcyclohexenone doesn’t come with much room for error. Anyone who’s spent time in a lab or plant knows the stakes. Vapors can irritate eyes and lungs. If it finds its way into groundwater, cleanup isn’t simple. Even a tiny slip-up can create hassle for staff and headaches for the community. That’s why smart storage makes more difference than folks think.
Why Storage Location Matters
Some people treat storage like an afterthought. From experience, that’s where problems start. Give chemicals like methylcyclohexenone their own spot. Spaces away from busy hallways or main work zones work best. A dry, cool room that always stays out of sunlight cuts down on evaporation and keeps containers safe. Sunlight speeds up chemical breakdown and plastics can weaken over time, so shield storage from direct rays.
Sticking with the Right Containers
This compound doesn’t get along with random plastics or old containers with rusty lids. Stainless steel or properly labeled, chemical-resistant drums do a good job. Always double-check compatibility charts before pouring anything new into an unknown vessel. I’ve seen spills that could’ve been avoided, if people took one last look at that chart hanging over the safety bench.
Labeling and Maintenance Go Hand in Hand
In any shop or lab, confusion comes from missing or sloppy labels. There’s no excuse for it. Use waterproof labels that say exactly what’s inside, with clear hazard symbols. It’s about protecting the next person who moves a drum or wipes a spill. Schedule regular checks so no leaks sneak up on you. Trust me, if a container leaks, it usually happens on a Friday afternoon or when the person who filled it is miles away on vacation.
Handling Risks from Static Electricity
Fumes from methylcyclohexenone ignite fast. Transferring the liquid? Ground and bond every container. This step sounds fussy until you’ve seen what a single spark can do. It’s not hard to connect a cable or check grounding straps. In fact, it keeps you from explaining accidents later.
Ventilation Earns Respect
Airflow sounds like a soft detail, but it changes everything. Store this compound in a spot that vents to the outside—always. Fume hoods and exhaust fans that work around the clock make it a lot safer for everyone. Even a low, steady leak can affect your health before you realize the smell has built up.
Training People, Not Just Writing Rules
People forget that training saves more money than fancy alarms. Bring new team members up to speed on how to store and handle methylcyclohexenone. Review steps every season. Hang clear instructions near the storage site—not hidden in a binder that gathers dust on a shelf.
More Than Following a Rulebook
Chemical storage policies shouldn’t just tick boxes for inspectors. Each guideline comes from something that went wrong for somebody, somewhere. Making a safe storage space respects the reality that one bad release can change lives, cost money, and land good folks in trouble. Up-to-date storage keeps doors open, lungs clear, and headaches out of your workday.
Understanding the Chemical
Methylcyclohexenone might sound like another tongue-twister from chemistry class, but it isn’t unfamiliar territory once you look past the name. This ingredient comes up in industries ranging from fragrances to specialty chemicals. It helps create certain scents, and from what’s been released in public records, it has managed to sneak quietly into a handful of products over the years.
Is It Dangerous to Health?
Most people, myself included, care a lot about what comes into our homes and bodies. Companies and watchdogs like the European Chemicals Agency have checked out methylcyclohexenone for years. Safety data tell us it can cause irritation if you spill it on your skin or get it in your eyes. Breathing in high amounts—such as during a big chemical spill—could cause dizziness, headaches, or sore throats. An old habit of mine, reading material safety data sheets before handling any chemical, proved its worth again after I checked out the latest one for this substance. Gloves and masks get recommended for a reason.
Real risks depend on how you use or contact this material. Workers at production plants or labs face more exposure compared to a person walking in off the street. While cases of methylcyclohexenone poisoning seem rare, lack of strong reporting doesn’t always mean everything’s fine. I’ve seen colleagues get careless with substances believed to be "low risk," only to regret it after a spill or splash.
Environmental Fallout
Chemicals tend to shed their secrets if you follow their path into water or soil. Studies hint that methylcyclohexenone doesn’t stick around long—it breaks down quicker than many industrial molecules. Even so, releases in large quantities can cause immediate harm to aquatic life. I learned the hard way during a university project; even a small chemical spill in a fish tank made the guppies struggle, reminding me that living systems react much faster than expected.
Hidden dangers often come from overlooked leaks or wastewater. Some countries require strict wastewater filtering. My visits to older factories showed why. It’s easy for a facility to discharge trace amounts into local streams, harming fish and insects, even when the chemical won’t linger for weeks.
Moving Toward Safety
No chemical tool is ever truly risk-free, and the story carries over here. Industry tools and watchdogs stress strong basic steps—good ventilation, regular monitoring, and up-to-date training for anyone handling methylcyclohexenone. These steps cost a bit up front, but I’ve seen them prevent hospital trips and fines.
Better research would help. Spotty data still leaves some health and environmental questions up in the air. Calls from advocacy groups for more transparent public reporting make sense after years of missing details. As a parent and citizen, I’d be uneasy if manufacturers skirted transparency or if disposal facilities scrimped on filtration. Surveillance and stricter penalties for companies that pollute—backed by real sampling and not just paperwork—would keep everyone safer.
Finding Balance
A chemistry lab isn’t a villain’s lair, nor is a chemical innocent just because it passes some safety checks. Methylcyclohexenone—like many lab discoveries—asks for respect. With the right handling, risks shrink fast, but ignoring warning labels brings trouble. Every safe choice we make now puts fewer worries on the next generation’s shoulders and gives us every reason to expect both healthy people and cleaner rivers.