Methyltrioctylammonium Chloride: Shaping Industry Through Chemistry
Historical Development
Methyltrioctylammonium chloride entered the chemical landscape during the mid-20th century, at a time when chemists began searching for more efficient phase transfer catalysts. The compound’s roots trace back to the innovation era following World War II, when basic research and industrial application often pulled in the same direction. Laboratories in both academia and industry worked with quaternary ammonium salts, seeking ways to move ions between water and organic solvents. By the 1970s, methyltrioctylammonium chloride became widely recognized for its ability to solve persistent separation problems. Early patents and studies mentioned this compound as a go-to choice for electrolytic extractions, indicating that the market didn’t just discover it—the industry needed it.
Product Overview
Methyltrioctylammonium chloride holds a place among quaternary ammonium salts—organic compounds known for their charge and versatility. Its three octyl groups attached to a nitrogen core, complemented by a methyl group and balanced with a chloride anion, give it unique properties. Chemists value it as a phase transfer catalyst, but that only scratches the surface. While competitors like tetrabutylammonium salts have their time in the sun, methyltrioctylammonium chloride boasts a longer alkyl chain, which translates to higher hydrophobicity. That shift affects where and how chemists use it: not just in simple transfer reactions, but in tasks where oil-loving behavior can make the difference between clean separation and a murky mess.
Physical & Chemical Properties
This salt looks waxy or solidified—sometimes a thick, sticky liquid at room temperature, depending on purity and ambient conditions. The color ranges from white to pale yellow. Solubility patterns draw a line: methyltrioctylammonium chloride dissolves easily in many organic solvents, including chloroform, dichloromethane, and toluene, but resists mixing with water. Melting points hover in the 40 to 50°C range, and density hovers around 0.89 g/cm3—both values chemists keep in mind before scaling up. The compound’s high molecular weight comes from those long octyl chains. Stability remains solid under typical laboratory storage, though exposure to strong bases or oxidizing agents leads to breakdown or reaction.
Technical Specifications & Labeling
Manufacturers must meet strict standards to market methyltrioctylammonium chloride for lab or industrial use. Purity, usually above 95%, needs to stay consistent. Detailed batch records, Certificate of Analysis, and Material Safety Data Sheets accompany each shipment—regulators demand it, and end-users rely on it. Labeling typically details the full IUPAC name, hazard codes, gross and net weight, recommended storage temperatures, and handling precautions. In the shipping world, classification as a hazardous material influences how it gets packed and who can handle it.
Preparation Method
Industrial syntheses start with octylamine and methyl chloride, moving through a series of alkylation reactions in organic solvents. Excess methyl chloride triggers the final transformation, pushing the tertiary amine to a fully quaternary state. Direct handling of methyl chloride gas—both toxic and hazardous—demands robust equipment and extensive training, facts that set responsible facilities apart from cut-rate operators. Workups often involve aqueous washing, solvent extraction, and drying under vacuum. Crude product sometimes needs recrystallization from alcohols or alkanes to reach required purity levels. Careful monitoring of pH and temperature throughout the process holds down side products.
Chemical Reactions & Modifications
The standout feature of methyltrioctylammonium chloride comes from the positive charge at the nitrogen center, balanced by the chloride anion. This structure allows the compound to shuttle other ions—fluoride, cyanide, sulfate—across immiscible boundaries between solvents. Chemists explore modifications by swapping out chloride for other anions, such as nitrate, perchlorate, or tetrafluoroborate, to change solubility or reactivity. Some researchers tweak the methyl or octyl groups, but usually the balance between hydrophobic and hydrophilic behavior dictates the choice. The compound’s robust nature means it doesn’t break down easily, so transformations focus on functional group exchange instead of structural overhaul.
Synonyms & Product Names
Other names might sound unfamiliar at first but describe the same compound: Aliquat 336 stands out as a prominent commercial name. Alternative chemical terms include trioctylmethylammonium chloride, methyltrioctylazanium chloride, or N-methyl-N,N,N-trioctylammonium chloride. Catalogs from Sigma-Aldrich, Merck, and lesser-known specialty suppliers may favor one moniker over another, but all refer to the core quaternary ammonium salt with four alkyl groups and one chloride counterion.
Safety & Operational Standards
Industry safety teams and laboratory personnel respect the hazards linked to methyltrioctylammonium chloride. It doesn’t ignite easily, but its toxicity lies in skin and eye irritation and possible long-term organ effects after repeated exposure. Nitrile gloves, splash goggles, and full ventilation represent the baseline. Spills call for absorbent pads and chemical waste protocols, not a quick sweep. Disposal falls under hazardous waste laws, and workers receive annual retraining on procedures. In shipping and storage, drums must stay sealed, upright, and labeled, well away from acids or bases. Regulatory guidelines trace back to classification by OSHA, REACH in Europe, and similar authorities worldwide.
Application Area
Real-world demand for methyltrioctylammonium chloride covers a surprising swath of industries. Hydrometallurgists use it to coax precious metals and rare earths from complex ores through solvent extraction. Wastewater treatment plants value it for breaking down stubborn organic pollutants that resist standard processes. Analytical chemists turn to this salt for phase transfer catalysis—moving anions from water into organic layers to speed up or enable specific reactions. Dye makers, pharmaceutical companies, and even some petrochemical firms keep its reagent jar on hand for its role in streamlining or enabling synthesis. That reach, earned over decades, comes from a proven track record rather than marketing.
Research & Development
Academic and industrial labs each carve out niches for new applications and process improvements. Some teams work on boosting the selectivity of metal extraction, aiming to reduce co-extraction of useless byproducts. Environmental engineers study how methyltrioctylammonium chloride carries organic contaminants into manageable phases, helping to remediate polluted groundwater. Others investigate greener alternatives to current solvents, hoping to pair phase transfer catalysis with lower environmental impact. Research budgets point increasingly toward lifecycle impact assessment, as companies must demonstrate they aren’t just efficient, but responsible. Grants, partnerships, and scientific conferences keep the conversation moving forward.
Toxicity Research
Toxicologists have tracked acute and chronic effects of methyltrioctylammonium chloride in animal models and cell cultures. Most studies point to moderate skin and mucous membrane irritation on direct contact. Careful dosing showed that large exposures—in particular, through inhalation or ingestion—lead to nervous system effects and some organ toxicity, but accidental poisoning remains rare in controlled workplaces. Regulators in the US, EU, and Asia keep tabs on published studies; they update exposure limits and reissue guidelines after every major finding. Newer research investigates the fate of the compound after environmental release, hoping to track breakdown pathways and residues in soil and water. The need for clear answers grows as use expands in resource extraction and industrial synthesis.
Future Prospects
The future for methyltrioctylammonium chloride looks shaped by both market needs and regulatory pressure. On the positive side, demand continues rising in green extraction technology, drug development, and advanced materials synthesis. Chemists and process engineers look for compounds that move ions and molecules efficiently, and methyltrioctylammonium chloride ticks the right boxes. At the same time, environmental watchdogs and workplace regulators push for better toxicity data and stringent limits on workplace exposure; their attention drives innovation toward safer handling and disposal. Researchers explore biodegradable analogs and closed-loop recycling methods, hoping to balance utility with responsibility. I see opportunities for anyone willing to link robust chemistry with meaningful stewardship of people and planet.
What Is It Doing in the Real World?
Most people probably won’t ever hear about methyltrioctylammonium chloride. You don’t see it in the cleaning aisle, and you’re definitely not tossing it into your coffee. But inside factories and labs, it tackles jobs nobody else really wants. It solves real headaches when folks try to move stuff between water and oil, which don’t like mixing.
Take extracting metals, for example. Getting rare elements out of rock or recycled electronics isn’t simple. Water grabs some materials, oil grabs others. Methyltrioctylammonium chloride jumps in and says, “I’ll handle the trade.” It grabs certain chemicals from the water and helps them hop into the oil phase, or vice versa. This makes the whole separation process smoother, which matters a lot when resources are getting harder to find.
Why Chemists Reach For It
So-called phase transfer catalysts help reactions go faster and work better. Without them, you’d need harsher conditions, more energy, and more waste. I watched a colleague waste hours trying to coax a product from a stubborn reaction, until someone brought out a tiny vial of methyltrioctylammonium chloride. The result– much better yield and less time waiting. For chemical engineers, shaving hours or even minutes off a process means money saved, less pollution, and a safer workplace.
Refineries, mining outfits, and chemical plants adopt compounds like this to help scale up their reactions. Industries making surfactants, plastics, pharmaceuticals—they need these helpers to push reactions that just won’t go under normal conditions. Even water treatment plants sometimes turn to this family of chemicals to separate out nasty stuff from the water supply.
The User’s Responsibility
There’s no ignoring the downside. Any chemical with power to move metals and drive tough reactions can cause trouble if folks don’t respect it. Handling means gloves, goggles, and serious containment plans. Spills can harm rivers and workers alike, since methyltrioctylammonium chloride doesn’t break down quickly if it gets out. I knew a researcher who learned the hard way—not double-checking a valve led to a stressful cleanup. Tight rules and good training change an industrial mess into a manageable risk.
I’ve seen responsible outfits prepare with safety data sheets, walk-throughs, and regular oversight. The push for greener chemistry led to efforts in swapping in safer alternatives, or at least tightening up how much is released into the air or drains. European rules, and now state regulations in the US, force companies to track and limit harmful spills. Data from environmental monitoring keeps the pressure on, flagging risk earlier.
Moving Ahead
The chemical industry always chases better ways to do old jobs, with less cost to workers and the planet. Methyltrioctylammonium chloride, for now, remains tough to replace in specific separations and phase transfer work. That explains the ongoing research: new catalysts, cleaner processes, and smarter recycling to capture every drop. Chemists, environmentalists, and regulators keep pushing to run cleaner. Small changes in a process—less waste here, safer storage there—stack up over years. As industry secrets become shared knowledge, safer and more efficient chemistry spreads.
In the end, this compound isn’t a household name, but it plays a key role in keeping vital materials available for electronics, batteries, and even clean water. With strict oversight and thoughtful innovation, the benefits can keep outweighing the risks.
Getting to Know Methyltrioctylammonium Chloride
Methyltrioctylammonium chloride doesn’t grab headlines the way some industrial chemicals do, but in chemical labs and certain manufacturing plants, it’s no stranger. This compound, better known in scientific circles as Aliquat 336, works as a phase transfer catalyst, helping scientists and engineers carry out reactions that would otherwise be tough in water-based environments. The question that keeps coming up isn’t its usefulness; it’s safety and health effects.
Health Risks: What the Science Reveals
Many folks working in research or factories share a simple concern: “Will this stuff make me sick?” Scientific data backs up the worry. Methyltrioctylammonium chloride can irritate the eyes, skin, and throat. Breathing in its dust or vapors increases these risks. Some studies on animals point to even deeper trouble at higher doses. The chemical may mess up cell membranes, leading to damage that’s more than skin deep.
Data from the European Chemicals Agency lists it as harmful if swallowed, causing severe skin burns and eye damage. So, there’s a strong case for protective gloves, goggles, and careful handling. As someone who’s handled chemicals in a lab, small mistakes with hazardous substances leave lasting lessons. I once splashed a lesser irritant on my hand while cleaning glassware. Even with quick rinsing, redness and discomfort lingered for days. With chemicals like methyltrioctylammonium chloride, carelessness can leave much bigger scars.
The Challenge in Industries
Factories and research labs value efficiency. Workers juggle speed with safety. Methyltrioctylammonium chloride speeds up chemical reactions that make products like pharmaceuticals, herbicides, and fine chemicals. But when strict safety practices come under pressure, exposure risks grow. Spills, vapor release, and accidental splashes can turn a regular shift into a medical emergency.
The chemical’s behavior in the environment adds another layer of concern. Reports from the U.S. Environmental Protection Agency hint at aquatic toxicity. If wastewater carrying these residues leaks into rivers or groundwater, local fish and other creatures may not stand a chance. Factories need to contain or neutralize wastewater before sending it down the drain. A lapse here risks fines from regulators and loses public trust.
Paths Toward Safer Workplaces
Training plays a huge part. Anyone working with chemicals, especially nastier ones, deserves clear instructions, not just a safety data sheet buried in a binder. Shortcuts and day-one slips often come from confusion or rushed hands, not malice. Supervisors and lab managers need to watch for warning signs—forgotten gloves, improper labeling—not just during inspections, but every day.
Better engineering controls help, too. Fume hoods protect against dangerous vapors. Spill kits, accessible within arm’s reach, can save situations that would otherwise spiral fast. It’s straightforward to roll eyes at “yet another safety rule,” but after seeing coworkers trip up, most people keep close ties with their gloves and eye protection.
Looking for Alternatives
Chemists often face trade-offs. Sometimes, methyltrioctylammonium chloride remains the best tool for the job. On other projects, safer substitutes—maybe a different catalyst or process—can cut the hazard by half or more. Industries serious about safety ask suppliers about greener chemicals and regularly reexamine their formulas. Government guidelines and pressure from major clients ensure this isn’t just wishful thinking—it’s business survival.
The Bottom Line for Workers and Communities
Even chemicals essential to modern production can bite back. Respect for methyltrioctylammonium chloride starts with good training, protective gear, and real oversight. Facilities using it owe workers and nearby neighborhoods open reports and real containment plans. Safer choices exist—it just takes the right spark to get an industry to shift.
The Real-World Story Behind the Chemical’s Requirements
Chemicals like Methyltrioctylammonium Chloride might sound obscure unless you work with them. I’ve spent time around labs and production sites, and I’ve seen careless habits turn small chemicals into big problems. So, it’s no surprise that this one needs special attention. Methyltrioctylammonium Chloride plays a role as a phase transfer catalyst in extractions and separations, and the way it gets stored has a lot to do with keeping both people and processes safe.
Why Moisture, Light, and Heat Are Real Threats
From what I’ve learned, this chemical stands up to normal conditions, but it reacts poorly if left out in the open. Even a few hours with the cap loose invites clumping and product degradation. Moisture in the air hits it fast, turning it clumpy or mushy. If you let light sneak in—a careless room lamp or daylight through a window—the chemical starts to yellow, which points to possible breakdown. Advanced labs mark their containers with strict storage rules: keep it dry, keep it shaded, keep it below ambient temperature. The difference between clear, reliable compound and contaminated goop is often a poorly closed lid or a busted desiccant packet.
Handling Practices from the Ground Up
I’ve seen people underestimate gloves, masks, or goggles because they think a solid or viscous liquid is less risky than a fuming acid. That notion doesn’t hold. If Methyltrioctylammonium Chloride gets on your skin, it can irritate and linger. A tidy storage locker with spill control materials on hand keeps accidents from spreading. Everyone should treat it with the same respect as the flammables and acids; that means personal protective equipment every single time. Safety data sheets say a small exposure isn’t disastrous, but no one wants tingling fingers or worse after a shift.
Why the Label and Date on Every Jar Matter
In practice, a shelf can hold a lineup of similar-looking bottles. Rushed workers grab the wrong one all the time. A clear, permanent label stops chaos before it starts. Adding a date signals when a batch arrived or first got opened—a simple trick to dodge the silent risk of degraded chemicals. Some folks still use a sharpie, but digital tracking proves better when inventory grows. At an industrial scale, digital logs and barcodes help avoid waste, financial loss, and environmental slipups.
Shared Spaces, Shared Responsibility
There’s no point in top-tier storage if the next shift doesn’t stick with the rules. Chemical management turns on trust and training. One team keeping up fails if others cut corners. Tidy shelves, regular walk-throughs, and a culture where someone calls out a cracked lid or an unlabeled jar help prevent issues before they hit. Shared responsibility works better than an endless stream of safety memos.
Building on the Right Approach
Some companies run drills for spills. Others hang best-practice posters above storage cabinets. Audits and regular retraining keep requirements clear. The goal always circles back to the same thing: control the environment and the risks drop. The chemical world doesn’t forgive laziness. Rules around storage and handling actually save money, protect workers, and keep the product right for the end use every time.
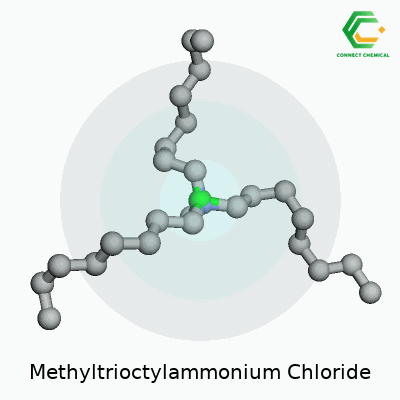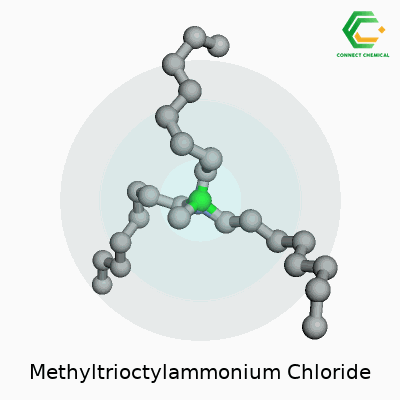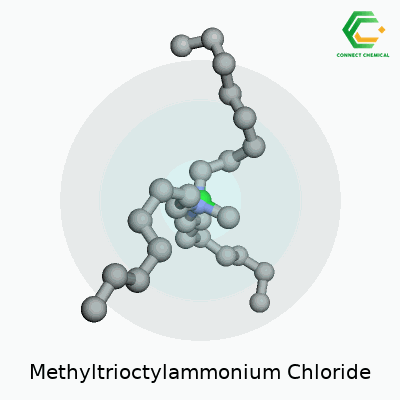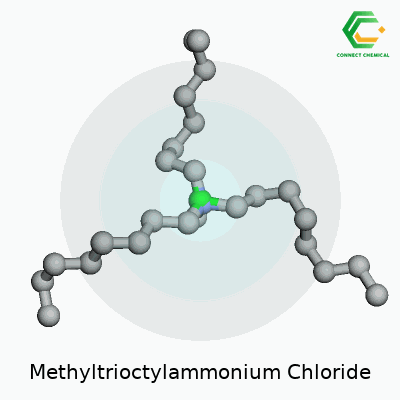
Chemical Structure and Formula
Methyltrioctylammonium chloride carries the formula C25H54ClN. This quaternary ammonium compound holds a methyl group and three octyl chains attached to a central nitrogen atom. The nitrogen has a fixed positive charge, balanced out by a single chloride anion. To paint a clearer picture, three long eight-carbon octyl groups extend from the nitrogen, giving the molecule a substantial hydrophobic character, while the methyl group finishes the fourth arm.
The structure looks like this: the nitrogen atom sits at the center, bonded to one methyl group (CH3) and three n-octyl (C8H17) chains, plus a Cl- hanging out as the anion nearby. In shorthand, the molecule often appears as [CH3(C8H17)3N]+Cl-. With a big hydrophobic section and a charge-packed head, it fits right into the world of phase transfer catalysts.
Why This Structure Matters
Anyone familiar with chemistry labs or industrial extraction knows that oil and water don’t mingle. Extracting metal ions, organics, or even drug precursors usually means mixing two layers that don’t want to talk to each other. Methyltrioctylammonium chloride changes the rules, using its long carbon arms to hang out in organic solvents while the charged nitrogen pulls ions out of water. The result: two worlds connect that normally repel each other.
In liquid-liquid extractions and phase transfer catalysis, this compound stands as a dependable choice because of its specific balance of hydrophobic and hydrophilic. Techs working in hydrometallurgy, for instance, rely on its ability to pull out precious metal ions from waste streams. The pharmaceutical industry finds value in its selective extraction power. Both cases boil down to what that structure provides: solubility in non-polar solvents, while anchoring inorganic ions long enough to drag them through the oil barrier.
Risks and Solutions
With those long alkyl chains, methyltrioctylammonium chloride doesn’t break down quickly in the environment. Persistence and toxicity concerns come up—as do stories of accidental releases downstream from old chemical sites. Cleanup costs rise when these compounds drift into waterways, so efforts are building toward greener quaternary ammonium agents or biodegradable alternatives without sacrificing separation performance.
Tracking and responsible handling play a big role here. Factories using this compound monitor storage tanks, install secondary containment, and review transport routines. Some firms turn to process changes that minimize spillage in the first place, such as using closed-system solvent extraction. Wastewater treatment plants dealing with run-off load up on activated carbon and special filtration beds to trap lingering molecules.
Anyone working in the field recognizes that change happens slow, but a growing awareness of environmental impact is moving research dollars into sustainable chemistry. Finding catalysts that do the same job—without sticking around for decades—is top of mind for university labs and chemical makers.
Looking Ahead
As industries push for cleaner processes, methyltrioctylammonium chloride shines a light on both the practicality of modern chemical tools and the importance of thinking through the full life cycle. Chemists appreciate what its structure achieves, but that same design prompts tough questions about fate, risk, and responsibility. Solutions won’t show up overnight, but new molecules, process tweaks, and accountability set the stage for progress.
Tracking Down a Specialty Chemical
Finding chemicals like Methyltrioctylammonium Chloride often turns into a hunt, especially for people outside major research or manufacturing. It’s not something most local hardware shops keep near the cleaning aisle. This kind of chemical usually serves specific uses in lab settings or for extraction processes in industry. With a name like that, you expect a certain level of background knowledge to just know what you’re looking for, much less how to buy it.
This compound shows up a lot in metal extraction, phase transfer catalysis, and sometimes in a few specialty ionic liquids. A student might only learn about it flipping through a dusty organic chemistry book. Somebody working in hydrometallurgy or rare earth separation, on the other hand, might see it every week. That difference shapes where you’ll have luck searching.
Routes for Purchasing Methyltrioctylammonium Chloride
Standard chemical suppliers make up the main path for anyone needing this chemical. Most people in academic or industry labs know Sigma-Aldrich, Alfa Aesar, and Fisher Scientific—these giants offer consistent supply chains, detailed safety sheets, and make it possible to double-check certificates of analysis. International options like TCI or Merck have websites that let researchers compare grades and pricing. A regular consumer probably won’t get past their account creation form, but organizations, universities, or registered labs can usually order directly.
Smaller specialty chemical suppliers sometimes pick up where the big names stop. Companies such as Toronto Research Chemicals or Spectrum Chemicals specialize in niche products. These stores target synthetic chemists or research labs. More online distributors, some of which focus on import and export, like Alibaba or Made-in-China, also list this compound. Many of those sources ask for a business license or end-use statement. International delivery comes with local regulations, extended customs holding, and extra paperwork, which some smaller buyers often overlook.
Every step along that chain demands safety protocols. A background in chemistry or a linked legal entity makes things smoother. Non-professionals bump into blocks rather quickly. Even experienced chemists often double-check storage guidelines or disposal rules because of strict regulatory pressure, especially following several high-profile chemical incidents that inspired lawmakers to tighten things up.
Why Sourcing Can Get Complicated
Legal hurdles exist everywhere, for sound reasons. Some countries treat Methyltrioctylammonium Chloride as a material with restricted uses given how it can be used to handle heavy metals or other substances needing care during research or disposal. In my own experience, even at a university, paperwork and purchasing clearance meetings around these sorts of chemicals never ended quickly.
Costs also run higher than bulk commodities. Anyone hoping to buy a small gram sample sometimes faces minimum order quantities, which leads to groups teaming up to split costs and quantities. That’s no small feat with expensive, shelf-sensitive compounds.
Shortcuts Don’t Pay Off
People should stay far away from unverified online sellers promising no-questions-asked delivery at a suspiciously low price. Counterfeit chemical sales have grown in the last decade, especially for rare or high-margin reagents. Incorrect compounds risk ruining experiments, wasting money, or causing genuine harm if handled incorrectly.
Safer practice relies on background checks, transparency, and traceability. Big suppliers keep tight control over the supply chain and documentation. Responsible buyers call references, ask for batch records, and check for third-party testing—even if that takes extra time.
Improving Access and Accountability
Easier ordering exists for established labs, but for those outside this circle, better transparency around approved distributors would go a long way. Professional organizations and regulatory bodies can maintain directories and even provide sample sourcing agreements. Governments can keep databases of vetted suppliers to filter out bad actors. Education also matters—training in procurement and chemical safety should run alongside rules about legal and disposal compliance.