Mucobromic Acid: Past Insights, Present Knowledge, and Future Uses
Historical Development
Curiosity often drives the earliest breakthroughs in chemistry. Over a century ago, researchers hunting for new organic acids stumbled across mucobromic acid during studies on brominated furan derivatives. The isolation and naming followed classic lab protocols of distillation, crystallization, and elemental testing. Records from the late nineteenth century mark its entry into chemical literature, tying its origins to experiments with bromine and mucic acid. Chemists soon recognized its potential for deeper organic synthesis work and for probing reaction mechanics involving halogen-containing compounds. The story of mucobromic acid tells us how a compound once tucked away in niche journals can end up in a surprising range of research pipelines.
Product Overview
Mucobromic acid presents itself as a crystalline powder, usually off-white or slightly beige. Its structure features a furan ring flanked by two aldehyde groups and high bromine content, which makes the architecture interesting for reactivity. Suppliers typically target academic labs and technical specialists, given the chemical’s specific applications and hazards. Packaging generally calls for light-proof amber bottles, sealed tightly to avoid moisture uptake.
Physical & Chemical Properties
Looking over a fresh sample of mucobromic acid, you see how the crystalline form and slightly acerbic smell signal its reactivity. Melting points hover near 150°C, with some variation related to purity and handling. The acid only slightly dissolves in water, but shows more solubility in polar organic solvents such as ethanol and acetone. Bench chemists know that bromine atoms elevate its density and increase its ability to participate in electrophilic addition and substitution reactions. The presence of both aldehyde groups and carboxylic acid cues careful handling, since stability changes under basic or oxidative conditions.
Technical Specifications & Labeling
Bottles often feature hazard labeling for strong irritants and environmental symbols reflecting regulatory controls on halogenated compounds. Typical commercial lots clock in at purity ranges of over 98 percent. Research-grade vendors list the precise batch analysis, moisture percentage, and contaminant screening results. Certificates of Analysis usually accompany shipments, listing molecular weight (276.85 g/mol), chemical formula (C4H2Br2O3), and the CAS number. Proper labeling guides safe storage and responsible disposal.
Preparation Method
I’ve run across mucobromic acid synthesis most often in graduate-level lab courses and some synthetic organic research. Preparation centers around a bromination reaction on mucic acid (itself a mild oxidation product of galactose). Concentrated bromine reacts with mucic acid in aqueous solution, forming mucobromic acid through a series of ring closures, condensations, and bromine additions. The resulting product requires filtration, repeated recrystallization, and sometimes column chromatography to hit high-purity marks for analytical work. No one forgets the sharp odor of bromine during these preparations, or the bright yellow-orange warnings on lab doors signaling active halogen chemistry inside.
Chemical Reactions & Modifications
Mucobromic acid plays both as a building block and a probe in organic synthesis. The bromine atoms and the ring-stabilized aldehydes act as reactive handles, opening pathways to form heterocycles, conduct nucleophilic substitutions, and test radical reaction mechanisms. In modification research, chemists often use mucobromic acid to synthesize furans, pyridines, and other aromatic compounds. Recent journal articles describe using it for the introduction of functional groups, creating intermediates in medicinal chemistry projects, and as a reference point for reaction kinetics studies involving dihalogenated intermediates.
Synonyms & Product Names
People have tried several names for mucobromic acid, reflecting both chemical structure and discovery roots: 2,3-Dibromo-4-oxobut-2-enoic acid, 3,4-Dibromo-2(5H)-furanone-5-carboxylic acid, and 3,4-Dibromo-malealdehydic acid among them. Some catalogues shorten it to DBMA for ease of reference. Knowing these synonyms eases literature and patent searches—and avoids confusion when different labs report findings under alternate titles.
Safety & Operational Standards
Mucobromic acid earns more red diamonds than most lab organics. It irritates skin, eyes, and the respiratory tract, and the material safety data sheets warn about its mutagenic effects in certain test organisms. European and American regulators both set strict exposure limits. I’ve always seen it handled in fume hoods, behind splash shields, with double gloves and chemical safety goggles. Environmental standards restrict waste streams containing mucobromic acid, pushing users to track and account for disposal using halogenated organic waste collection only.
Application Area
Talk of mucobromic acid often stays between organic chemists, pharmaceutical researchers, and a few agrochemical innovation teams. Its reactivity shines most in pathway exploration for complex molecule synthesis. The high reactivity of the dibromo-furan core supports its use as a precursor for many other compounds that end up as dyes, biologically active molecules, or structural probes. Teaching labs occasionally introduce it in advanced organic coursework to challenge students with multi-step synthesis and product purification.
Research & Development
Recent research efforts span multiple angles. Journals in organic chemistry keep publishing on mucobromic acid’s usefulness for creating new building blocks, especially for drug discovery involving brominated heterocycles that mimic biological structures. Material science researchers investigate its potential for polymer precursors and specialty coatings where controlled reactivity matters. Most R&D work concentrates on using the acid to create complex ring systems, and to test selective catalytic reductions, with a trend toward green chemistry and process intensification.
Toxicity Research
Toxicologists flag mucobromic acid as a substance worth caution rather than panic. In animal studies, it shows evidence of DNA damage and cytotoxicity at moderate exposure levels. Assays reveal oxidative stress as a probable cause. Regulators responded with classification as an irritant and potential mutagen, mandating strict exposure monitoring in the workplace. Environmental toxicology highlights its potential to harm aquatic life, demanding careful handling of even small waste volumes. Training courses for lab workers now standardize protocols for accidental exposure and reinforce risk awareness, not just for the individual, but for any process that might leak fumes or generate dust.
Future Prospects
Future paths for mucobromic acid look tied to digital chemistry and information-driven research. Data mining from high-throughput screening campaigns often turns up brominated heterocycles as promising leads, leading to increased scrutiny of building blocks like mucobromic acid. As computational chemistry refines prediction of chemical behavior, I expect more tailored uses for mucobromic acid derivatives in medicinal chemistry, advanced material design, and electronic component synthesis. Sustainability now anchors most industrial research, so green preparation methods—using less hazardous solvents, reducing waste, and recapturing bromine—stand out as key focus points. Innovations in process automation and in-situ reaction monitoring can help minimize human exposure and environmental impact, opening new opportunities for this niche but powerful acid to shape science in raw and refined forms alike.
Practical Use in Laboratories
Walk into any well-equipped organic chemistry lab, and odds favor a shelf holding a bottle labeled "mucobromic acid." Chemists prize this compound for what it brings to the table during synthesis and analysis. Its magical quality lies in a strong reactivity, especially in creating carbon–carbon bonds. That matters deeply for anyone exploring new molecules or screening medicinal compounds.
What jumps out about mucobromic acid is its use as a building block. It often ends up in the hands of researchers working on heterocyclic chemistry. That’s a category with plenty of scientific sparkle, since heterocycles form a backbone for loads of drugs, dyes, and even some polymers. Over the last decade, papers have highlighted how scientists rely on mucobromic acid to add pieces like pyridones and furan rings to new substances. That isn’t just a curiosity—it shapes the search for treatments and novel materials.
Spotlight on Medical and Pharmaceutical Research
Mucobromic acid stands out as a tool when testing the reactivity of nucleophiles in a laboratory setting. Nucleophiles matter for how drugs get built in the first place. Early medicinal chemists used to face tough odds making new scaffolds. This organic acid bent those odds a little, by serving as a starting point for dozens of molecules that later showed antimicrobial, anticancer, and anti-inflammatory promise. Scientists noted, for instance, that when they modified the bromine atoms on the molecule, the products often turned out to be better at fighting bacteria in test tubes.
Efforts in early-stage drug discovery often rely on compounds like mucobromic acid to "stress-test" synthetic pathways. Before a new drug makes it to the pharmacy shelf, it goes through a gauntlet of reactions to prove safety and effectiveness. Every year, researchers publish studies describing how mucobromic acid allows them to investigate new reactions or build rare structures that could—someday—serve as medicines. Not many outside the industry hear about this behind-the-scenes labor, but it quietly fuels the next wave of pharmaceutical breakthroughs.
Environmental Chemistry and Detecting Pollutants
Beyond making molecules, mucobromic acid pops up in environmental science circles. That might sound strange for an organic chemical, but the reason links back to water safety. Chlorinated and brominated byproducts, including mucobromic acid, can form when water gets disinfected. Specialists in environmental chemistry track these byproducts to ensure drinking water stays safe, measuring parts per billion and chasing even tiny traces. Reports from the U.S. Environmental Protection Agency underline why such vigilance counts—long-term exposure to disinfection byproducts can affect public health in subtle but meaningful ways.
Looking Toward Safer Handling
Mucobromic acid isn’t a chemical to take lightly. Its reactive nature translates to possible risks. Researchers and lab workers learn early to respect safety data: use a fume hood, glove up, avoid skin contact. Why such care? Studies found certain compounds related to mucobromic acid irritate the eyes, skin, and respiratory tract, and some forms raised red flags about mutagenic potential. As with any strong reagent, mistakes lead to trouble. Sharing safety information and training new chemists brings those risks down.
Potential for Greener Chemistry
Plenty of chemists look at mucobromic acid with appreciation—but also with an eye for sustainability. Some suppliers already focus on producing it with fewer hazardous byproducts and minimal waste. Green chemistry remains an active field, and using renewable feedstocks or smarter reaction conditions could push mucobromic acid into the next era of responsible research.
Understanding the Make-Up of Mucobromic Acid
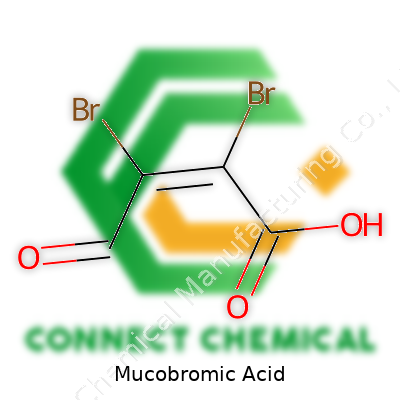
Ask any chemist who’s spent time with halogenated compounds, and mucobromic acid will come up sooner or later. Mucobromic acid carries the formula C4H2Br2O3, giving away its penchant for complexity and reactivity. It sports two bromine atoms, a couple of carbonyl groups, and an unsaturated furan ring. This isn’t a casual player—its double bonds and halogens put it squarely in reactive territory. Its full structure features a five-membered ring with the bromines attached at the 3 and 4 positions, an aldehyde group at the 2-position, and a carboxylic acid at the 5-position. Name aside, in the flask it looks like pale yellow needles with a tendency to demand respect for both its chemistry and toxicity.
Lab Experience with Halogenated Furans
I’ve had only one up-close encounter with mucobromic acid, but I’ll never forget the way it stings your nose. The reactivity comes down to its unique arrangement. Combining electron-withdrawing bromines on the furan backbone makes it a potent electrophile. That’s why researchers keep it under a hood and label it well—handling without proper protocol risks burns and long-term exposure. Many in the lab reach for this acid in synthetic work, especially for constructing complex heterocyclic scaffolds or for use as a building block in medicinal chemistry. Fast reactions, vivid color change, strong odor—classic signs of a functional group-rich molecule.
Why Mucobromic Acid Matters in Research
For those working in heterocyclic chemistry, mucobromic acid isn’t just another halogenated compound; it’s a valuable tool. The presence of the furan ring with its reactive positions lets chemists explore routes not open with simpler acids. Those twin bromines offer easy sites for further modification through substitution, arylation, or cross-coupling. Published research highlights its role in creating antitumor and antiviral agents. Its chemical nature also helps map interactions with biological macromolecules, a step that often determines the success of drug development projects.
Staying Safe in the Lab
No matter how valuable a compound might be, safety means more than just gloves and goggles. Mucobromic acid’s pungency signals its hazard profile—skin burns, respiratory risks, and even carcinogenic potential call for extra vigilance. Running its synthesis or handling its solutions means having access to reliable fume extraction, proper containment, and emergency protocols. I’ve worked with plenty of reactive aldehydes over the years, but few demand attention like mucobromic acid. Documentation, clear labeling, and storage in appropriate glassware keep both the researchers and the experiment running smoothly.
Practical Solutions for Handling and Waste Management
Every step with mucobromic acid, from weighing out the solid to cleaning up the last trace, benefits from careful planning. Labs that see regular use of this compound train staff to recognize the smell and practice spills response. Waste streams separate out halogenated organics for proper disposal. Sharing real world tips—like working in small batches and storing stocks in the cold with ample desiccant—helps labs avoid common pitfalls. The structure’s complexity supports its strength, but it demands respect for the risks it brings.
What Makes Mucobromic Acid Worth Careful Handling
Mucobromic acid, a chemical known for its strong oxidizing power and reactivity, often finds its way into labs and research spaces. Anyone who has worked around it knows just how unforgiving it can be if overlooked. The substance reacts fast with moisture in the air, triggering hazardous fumes and degrading in quality before you know it. No responsible chemist leaves mucobromic acid on a shelf without taking some thoughtful measures to keep the compound safe.
Understanding the Hazards: Not Just Another Lab Supply
Many chemicals out there pose risks — mucobromic acid stands out by attacking human tissue and releasing toxic gases if mishandled. Touch or inhale it, and you’re looking at damage that could send anyone straight to medical care. Over time, tiny leaks or exposure to a humid room can damage the container, ruin the product, or threaten the safety of anyone nearby. The stories shared by lab techs all carry a strong message: never cut corners with this one.
Getting Storage Right: More Than a Label On the Jar
A dry, tightly sealed environment changes everything. Glass bottles with Teflon-lined caps won’t react with the acid, and these containers can stand up to the compound’s aggressive nature. Placing the bottle in a desiccator or a dry box shields it from ambient moisture, stopping hydrolysis before it starts. Many seasoned researchers keep their mucobromic acid in a cool, temperature-stable space, often somewhere below ambient room temperature, but not frozen. Not freezing is key — if condensation forms after thawing, the material risks partial degradation and loss of potency.
A Place Away From Light and Heat
Direct sunlight can trigger decomposition reactions, so leaving this compound near a window or under intense lights speeds up its expiration. Storing it in an amber glass bottle adds another layer of defense against harmful UV rays. Warm rooms or areas near heating equipment should always be avoided. Colleagues storing mucobromic acid in climate-controlled chemical cabinets prove that temperature consistency slows down the subtle breakdown processes that can otherwise go unnoticed for weeks.
Clear Labels, Smart Placement
Crowded chemical cabinets don’t just block fresh air — they invite confusion. A clearly labeled bottle with hazard warnings helps staff quickly identify and handle the substance responsibly. Storing mucobromic acid far from bases, oxidizing agents, and other reactive compounds slashes the risk of dangerous interactions, as even a splash or spill can result in almost instant chemical reactions.
Training and Review: Not Just a Box to Check
Staff must keep up-to-date with best practices. A quick refresher on critical storage habits prevents slip-ups that can turn ordinary routines into emergencies. More than once, annual reviews have caught cracks in glassware, failing desiccators, or outdated labels. Regular checks extend shelf-life and reinforce the culture of safety that keeps people and research intact.
Simple Steps, Big Impact
In my own work, the effort spent on proper storage has prevented mishaps more than fancy equipment alone. A dry space, solid container, clear label, and up-to-date knowledge — these practical steps protect people, assets, and the science at stake. Anyone who takes these to heart lowers risks, saves money on spoiled stock, and keeps a clear conscience where lab safety is concerned.
Understanding the Risks Behind Mucobromic Acid
Mucobromic acid doesn’t ring many bells for the general public, but chemists and lab workers pay close attention to its name. This compound sits in a class of organic bromides. Even from my own limited lab time, I remember those brown-red solutions—always a warning sign for something reactive. Many lab manuals mark mucobromic acid with hazard warnings, and after glancing through them, it’s pretty clear why. Its reactivity can surprise the unprepared.
A bit of digging through research journals and chemical safety sheets turns up sharp warnings. Contact with mucobromic acid can irritate the eyes, nose, and throat. It bites into skin and mucous membranes, and when inhaled, it may cause coughing and difficulty breathing. The acid can also bring on headaches, nausea, or worse, especially if a larger amount enters the body. There’s even some evidence showing DNA damage and a possible cancer risk. Animal studies have shown changes in cells after exposure. That’s nothing anyone should ignore.
Why Handling Precautions Matter
Day-to-day, most people never encounter mucobromic acid. People in chemical research or specialized industry work are the folks most likely to run into it. In my time helping prep school chemistry kits, all compounds like this went straight into the hazardous bin, far from where teens could get into trouble. Even a seasoned chemist will put on goggles, gloves, and a fume hood as the first line of defense. The acid reacts badly with water, acids, and bases. It takes just one accident to remind everyone what’s at stake.
Many chemical companies label and control mucobromic acid as a hazardous substance under workplace safety laws. The Occupational Safety and Health Administration (OSHA) and European Chemicals Agency (ECHA) both flag this acid as dangerous. There’s no debate among experts—the material deserves respect and careful handling. Disposal overseen by trained staff minimizes harm, while old-school practices like washing materials down the sink no longer cut it.
Facts and The Path Forward
Toxicity sticks to mucobromic acid for two reasons: short-term effects on the body and longer-term worries about chronic exposure. Acute symptoms hit fast, but cancer risk and genetic harm take years to show up. Lawmakers and regulators stay watchful for new evidence of damage. Industry guidance sometimes lags behind. Education offers the best protection. If you work in a lab or teach chemistry, ongoing safety training helps prevent careless shortcuts. Recent years brought better personal protective equipment and improved ventilation systems. Old labs sometimes still lack the right setups, so retrofitting them should be a priority.
Substitution plays a big role in chemical safety. For routine educational experiments, finding an alternative to mucobromic acid brings clear benefits. Chemicals with safer track records already replace it in some schools. This sort of shift depends on continued investment and smart shopping by department heads.
The health risks from mucobromic acid are real and supported by research. People working with or around it need solid information, proper training, and a culture of safety. Governments and institutions have set many rules to curb unnecessary risks, but real safety only works when everyone takes the issue seriously each time.
Clear-Cut Appearance and Texture
Mucobromic acid stands out as a pale yellow to light brown crystalline solid. At room temperature, it tends to form needle-like crystals, catching the light in a way no powder ever does. This material dissolves fairly easily in water, sending up an acrid, sharp odor. The scent gives a clear warning: handle it with care. Years working with chemicals in university labs taught me that respect for such warning signs pays off in the long run.
Stability and Handling
Store mucobromic acid in a cool, dry space away from direct light. It holds up under standard laboratory conditions, but hot and humid storage can prompt it to break down. A tightly sealed container slows down exposure to air and moisture. The first time I handled mucobromic acid, my mentor stressed keeping all lids on and working under a fume hood, since the vapors sting both nose and eyes. Over the years, workers in both academic and industrial settings have relied on simple controls: gloves, goggles, and good ventilation.
Melting Point and Solubility
Mucobromic acid melts right around 126°C. The range does not leave much room for error; a basic hot plate can push it past its melting point in moments. Dropping the temperature back down, the acid crystallizes out of solution in clear needles once more. This temperature window matters. Chemists depend on reliable melting points for purity checks. A batch that strays from this point often hides impurities that can change both behavior and safety risks.
Its solubility in water sets it apart from many halogenated organic solids. Add just enough water, and the orange-yellow solution forms. This water-loving nature comes from the carboxylic acid group in its structure, which finds friends in water molecules. Ethanol and acetone also take up mucobromic acid, though not quite as eagerly as water. This ease of mixing helps researchers tweak concentrations for reactions or extractions.
Dangers With Direct Contact
Touching mucobromic acid with bare skin burns quickly. Even a drop leaves redness. Its crystals act as a strong irritant, confirming published safety data. Safety sheets peg mucobromic acid as a toxic substance, with mutagenic and carcinogenic potential noted in literature. Years in the chem lab made safety second nature: never skip gloves with mucobromic acid, never set it near food or skin, and wash spills away fast.
Practical Implications and Solutions
Research teams often use mucobromic acid as a building block for complex molecules, so reliable information about its solid and liquid forms matters a great deal. I worked on a synthesis where a brief exposure to moisture set progress back several days. Reliable fume extraction, airtight storage, and clear labeling turn that kind of setback into a lesson, not a disaster. The world relies on people enforcing simple, clear safety protocols—goggles, PPE, and time in the hood.
Making substances like mucobromic acid safer for those using them may come with better public sharing of safety concerns, better workplace training, and regular hazard reviews. Rules get written after problems; experience often means remembering how following them felt, even when it slowed down the work. Chemical properties aren’t just trivia for textbooks: they set the rhythm of safe handling, smart storage, and successful lab work.