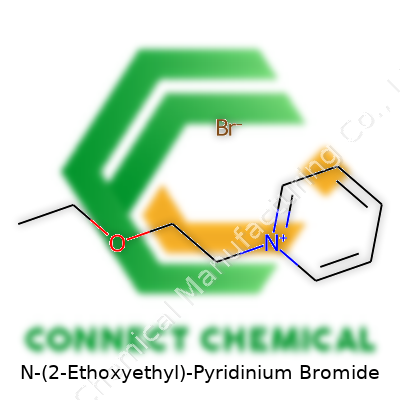
N-(2-Ethoxyethyl)-Pyridinium Bromide: Perspective on a Modern Chemical Intermediate
Historical Development
Chemical research has always leaned on creative salts and ionic compounds to solve tough synthesis problems. N-(2-Ethoxyethyl)-Pyridinium Bromide built its reputation as researchers searched for more versatile phase-transfer catalysts and ionic liquids starting in the twentieth century. Early literature in organic and medicinal chemistry journals from the 1960s mentions pyridinium compounds in exploratory alkylation reactions, but adding the 2-ethoxyethyl group opened the door to better solubility and less volatility than short-chained analogues. Academic chemical synthesis labs favored these modifications for nucleophilic substitution and as quaternized intermediates. The growth in ionic liquids and green solvent alternatives in the late 20th century kept pushing research into new pyridinium salts, cementing N-(2-Ethoxyethyl)-Pyridinium Bromide’s spot in catalogs and reference syntheses.
Product Overview
If you work with complex organic synthesis, you’ve probably run into this salt. Suppliers list it among phase-transfer reagents, ionic liquids, and specialty intermediates. Lab benches stack small glass bottles filled with its white to off-white crystalline powder. It offers a balance: easy enough to handle, soluble in water and some organics, neither too reactive nor too sluggish. Chemists use it in catalytic roles, quaternization, or to prepare more elaborate heterocycles and ligands. The product often carries a research-grade label—enough purity for advanced organic or pharmaceutical work, but not so costly or rare that it’s reserved for only the largest facilities.
Physical & Chemical Properties
Solid at room temperature, N-(2-Ethoxyethyl)-Pyridinium Bromide usually forms a white, sometimes faintly yellow, crystalline powder. Density comes in noticeably higher than many organic solids due to the bromide content. Melting point ranges between 160-170 °C for most high-purity batches, although trace impurities and humidity push this figure a few degrees up or down. The salt dissolves well in water and polar organic solvents—methanol, ethanol, and even warm DMF pull it into solution, which unlocks its potential in both aqueous and organic-phase reactions. Chemists familiar with its behavior know moisture control matters; hygroscopic samples clump fast under humid conditions and demand tight capping.
Technical Specifications & Labeling
A chemical received without proper labeling causes headaches for everyone in the lab. Reliable suppliers ship N-(2-Ethoxyethyl)-Pyridinium Bromide with batch-specific purity—sometimes quoted as ≥98%. The label gives a molecular formula, C9H14BrNO, and the molecular weight, 232.12 g/mol. Many bottles display a lot number and sometimes a synthesis route, which builds confidence in the lab’s chain of custody. Larger industrial packages add storage guidelines—“store below 25°C,” “protect from light”—to avoid decomposition or caking.
Preparation Method
The classic route to N-(2-Ethoxyethyl)-Pyridinium Bromide starts with pyridine and 2-ethoxyethyl bromide. Mix them under controlled cooling to minimize exotherms—this reaction moves fast, heating the mixture if not watched closely. Stirring in an inert solvent like acetonitrile, the reaction steers toward complete quaternization in a matter of hours. Recrystallization from ethanol or isopropanol strips away side-products, leaving the target salt as a filtered powder. Some researchers optimize yield by slow addition of the alkylating agent and careful purification, especially when scaling up. Modern microwave-assisted synthesis trims time and avoids some old bottlenecks, but the basics haven’t changed much in decades.
Chemical Reactions & Modifications
Organic chemists look to N-(2-Ethoxyethyl)-Pyridinium Bromide for its solid leaving group ability and stable pyridinium core. It swaps the bromide for other nucleophiles without fuss, setting up downstream ligands or serving as a launching pad for more elaborate heterocyclic frameworks. Researchers modify the 2-ethoxyethyl group to tune solubility or reactivity, adding bulk or branching to suit a wider toolkit. In multi-step syntheses, it acts as a quaternizing agent for nitrogens elsewhere in complex molecules. It even finds use in preparing task-specific ionic liquids—change the pyridine ring or swap out the alkyl chain, and a family of related compounds emerges, each serving in solvent or electrolyte design.
Synonyms & Product Names
Catalogs and regulatory lists keep N-(2-Ethoxyethyl)-Pyridinium Bromide under a few alternate names. The main ones are 1-(2-ethoxyethyl)pyridinium bromide and Pyridine, N-(2-ethoxyethyl)-, bromide. Specialty vendors sometimes register tradenames, but industry discussions orbit the standard chemical nomenclature. CAS Number labels (like 41520-28-3) add another layer—search one, and you track down the same compound regardless of branding.
Safety & Operational Standards
Every lab professional eyeing this salt pulls the SDS right away. Even though N-(2-Ethoxyethyl)-Pyridinium Bromide doesn’t throw off toxic vapors, direct contact causes mild skin or eye irritation, so gloves rule the day. Dust control and local ventilation matter during weighing and transfers. Practical chemists respect its modest flammability, especially since organic solvents used in synthesis increase fire risk. Waste must avoid drains; local regulations demand solvent or salt mixture collection for proper disposal. Standard practice includes labeling syringes and spatulas to avoid cross-contamination. Regular chemical hygiene—goggles, closed shoes, and sealed storage—keep incidents rare. Quality control teams test each lot for heavy metals or residual reactants, minimizing unknowns for users.
Application Area
Organic synthesis and catalysis absorb most of the world’s supply, but its role in chemical research is broader. It serves in the fabrication of ionic liquids for electrochemistry, helping scientists push the boundaries in batteries and supercapacitors. In analytical chemistry, it works as an ion-pair reagent or a phase-transfer catalyst. Pharmaceutical chemists sometimes include it for drug precursor synthesis, particularly in complex alkaloid routes or heterocycle construction. Environmental testing teams use related salts to calibrate sensors or act as standards in trace analysis. In academic settings, one finds it in educational demonstrations of electrophilic substitution or ionic structure-function relationships.
Research & Development
Labs focus on structure-activity relationships, trying new modifications on both the pyridine core and the alkyl side chain to squeeze out better solubility or conductivity. Electrochemists look for new applications in redox flow batteries and ionic conduction membranes, where traditional solvents and salts fell short. N-(2-Ethoxyethyl)-Pyridinium derivatives occasionally break into enzyme inhibition or radiolabeling pathways in biomedical research. Green chemistry pushes toward safer, recyclable ionic liquids—here, researchers use the core salt as a platform to bolt on biodegradable or more environmentally benign fragments. Leading-edge patents track improvements in phase-transfer catalysis, looking for reaction conditions that slash side-product formation and energy consumption.
Toxicity Research
Animal data stays limited, but toxicology screens pin its acute toxicity in a range similar to many lab-scale organic salts. The lack of strong, long-lasting toxicity is good news for researchers, though repeated exposure or large accidental doses carry risks judged by standard lab safety protocols. Mutagenicity and carcinogenic potential remain unproven at low exposure levels, but routine handling with gloves and ventilation cancels out most user hazards. Regulatory authorities haven’t flagged it as a major threat, but prudent researchers monitor every new toxicology report, keeping an eye on possible occupational hazards in case of chronic exposure.
Future Prospects
Demand for better ionic liquids, new phase-transfer catalysts, and advanced electrolyte components keeps interest in N-(2-Ethoxyethyl)-Pyridinium Bromide steady. Researchers tinker with both the ring and the ethoxyethyl chain to deliver greener, less toxic, or more recyclable variants for battery and solvent technologies. Academic-industrial partnerships pave the way for new synthesis routes that cut energy usage or hazardous by-products. As the world leans harder on green chemistry, waste minimization, and renewable feedstocks, expect new derivatives to attract industrial attention. The basic compound keeps its place as a useful standby, helping labs streamline syntheses as they push toward safer, more sustainable chemistry.
Understanding the Compound
N-(2-Ethoxyethyl)-Pyridinium Bromide may sound like something that belongs in a niche science journal, but this chemical finds value across a few practical corners of chemistry. In labs, it's often called a phase-transfer catalyst or a building block for more complex compounds. The interesting part comes from its structure—pairing a pyridinium ring with a two-carbon ethoxy side chain and a bromide counterion. This design gives it some unique properties that chemists appreciate, especially when reactions need a boost crossing from water to organic solvents.
What Does It Do?
I’ve worked on synthesis projects where moving ions between phases becomes a real headache. That’s where N-(2-Ethoxyethyl)-Pyridinium Bromide steps in. As a phase-transfer catalyst, it helps ions travel from water-based environments to oily, organic ones. Without that help, you might end up with a stalled or incomplete reaction—a waste of both time and materials. This compound actually opens doors for new reaction pathways that would otherwise never happen. In everyday lab practice, this means more efficient routes to pharmaceuticals, plastics, dyes, and specialty chemicals.
Why Does It Matter?
Getting reactions to happen more smoothly cuts down on the need for harsh chemicals and extreme conditions. Many older reactions used heavy metals or high temperatures that created waste or safety risks. With N-(2-Ethoxyethyl)-Pyridinium Bromide, researchers can sometimes use milder conditions with better results. This isn’t just good for the people handling the chemicals—it’s a win for the environment and anyone living downstream from a chemical plant.
Researchers also use this compound to prep advanced materials, such as ionic liquids. Some teams working at universities have published papers showing how small changes to the pyridinium structure can shift electrical and physical properties in interesting ways. In my experience, tweaking these types of compounds can deliver big improvements for battery research and conductivity experiments. You might not see a bottle of this in your medicine cabinet, but the benefits ripple out to everyday electronics and cleaner manufacturing.
Challenges and Responsible Use
Like every tool, N-(2-Ethoxyethyl)-Pyridinium Bromide deserves respect. It’s not food-safe, and spills or misuse in the lab can cause real harm. Bromides can be irritating, and pyridinium salts demand proper handling. Anyone using this compound should lean on established safety protocols, wear gloves and goggles, and stay aware of ventilation needs. I remember a lab partner who ignored these steps—he ended up with a nasty rash and a formal write-up. Safety rules exist for a reason.
Finding Pathways to Safer and Greener Chemistry
Green chemistry is no longer a wish list item; it’s a necessity. So the next steps would involve improving how we use N-(2-Ethoxyethyl)-Pyridinium Bromide—recycling it where possible, designing processes that generate less waste, and searching for alternatives that offer the same catalytic value with even lower risk. I’ve seen teams use water-based catalysts and renewable feedstocks to cut reliance on harsher chemicals. These innovations started out as lab-scale curiosities, but some now shape entire production lines at forward-thinking companies.
In the big picture, compounds like N-(2-Ethoxyethyl)-Pyridinium Bromide show how thoughtful chemical work can improve efficiency and safety in ways that matter far outside the lab. With care, collaboration, and commitment to progress, the lessons learned from these molecules can keep chemistry moving in responsible directions.
What’s Inside the Name
N-(2-Ethoxyethyl)-pyridinium bromide might sound like something from a pharmaceutical lab, but its chemical foundation speaks clearly. This compound’s formula is C9H14BrNO. Every chemist reads that and sees the backbone of pyridine (C5H5N), then the side chain carrying an ethoxyethyl group, then the bromide counterion. For those comfortable with organic chemistry, the formula reveals a picture: a nitrogen-containing ring—pyridine—attached to a 2-ethoxyethyl group, carrying a positive charge and balanced by the bromide anion.
Why Chemical Formulas Matter
In my early days of lab work, one of my mentors drilled into me that knowing a compound’s structure gives a huge advantage. If you handle N-(2-Ethoxyethyl)-pyridinium bromide, just seeing C9H14BrNO on a bottle instantly hints at its molecular weight, charge, and solubility. Most chemists spot the nitrogen’s positioning and the presence of bromine and sense that this compound will mix well in polar solvents, impossible for many students to grasp without cracking a book. Yet once you’ve tried to dissolve something in water or acetone and failed, the true value of a formula clicks into place.
The presence of the pyridinium ring also points to unique reactivity. These structures can act as phase-transfer agents, sometimes used in organic synthesis to shuttle ions between layers, similar to how a doorman lets the right people through at a club. This trait comes straight from the way the nitrogen sits in the ring and the fact that the whole structure carries a permanent positive charge, kept neutral overall by the bromide sitting nearby in solution.
Applications, Hazards, and Down-to-Earth Lessons
Take a closer look at this salt, and the real-world impact feels much closer. Research labs sometimes reach for pyridinium salts like this one to modify nucleophilicity in chemical reactions. They help create better yields in reactions where a boost or control is crucial. The trick lies in chemical intuition, often honed through error. Knowing the formula, chemists anticipate possible byproducts or hazards. With bromide in the mix, there’s always some concern about its environmental effects and toxicity. Bromides can be relatively benign, but in bulk or under the right conditions, they present problems you can’t ignore.
In my own work, just glancing at the formula sparks a checklist: Will this compound produce dust? Should gloves be thicker than usual? Will a reaction dump bromide waste into drains? Awareness grows over time, and these questions turn into safer and smarter handling. The environmental issue runs deeply because waste bromides shouldn't end up in rivers. Labs have a duty to neutralize, recycle, or reduce their chemical footprint—something all chemists face at some point or another.
Building on Knowledge with Practical Steps
Solutions exist on both an individual and community scale. Chemists with experience learn to limit use, opt for less harmful reagents when substitution works, and support collective waste-handling strategy. Institutions do their share by training staff, providing safety data, and ensuring disposal follows regulation. Some communities have shifted towards green chemistry, pushing for alternatives with less persistent halide waste. For any lab, it's a win to plan for minimal runoff and find greener reaction conditions without sacrificing research quality.
Examining N-(2-Ethoxyethyl)-pyridinium bromide through the lens of its formula, you catch sight of a bigger picture: each element and group brings benefits and risks. The real skill grows from respecting the power behind the structures we handle—and making better choices, step by step, both inside and outside the lab.
Knowing the Material
N-(2-Ethoxyethyl)-pyridinium bromide doesn’t always ring a bell unless you’ve worked in a lab or a chemical supply room. Like many specialty chemicals, this compound steps outside the territory of household names, but it comes with the same core responsibility – keep it stable, and keep yourself safe. I learned early in my science days that skipping a few storage steps can lead to degraded samples, wasted money, and health hazards nobody needs in their day.
Best Spot for Storage
A cool, dry place works best. In my own experience, chemicals lose their punch or get weirdly clumpy if you keep them in humid rooms. Even worse, some materials will break down fast when exposed to sunlight or warm temps. Cabinets marked for chemical storage give the right protection. If you’ve got a refrigerator set aside for lab use, not mixed up with your lunch, that’s where many researchers keep moisture-sensitive compounds. No snacks allowed—cross-contamination risks just aren’t worth it.
Direct sunlight can speed up all kinds of chemical reactions. I’ve seen yellow powders darken after only a few days on a windowsill. Fluorescent room lights sometimes affect sensitive chemicals too. We wrapped bottles in foil or tucked them in opaque bins to keep them protected.
Sealing and Labeling
A snug cap prevents evaporation and contamination. Tiny things like lint or a drop of water from the air introduce surprises over time. I’ve thrown out more than one reactive bottle that picked up moisture. Good habits mean always checking seals after use. Mixing lids – even if you’re sure – creates confusion, and confusion in a lab rarely ends well.
Labels matter as much as the seal. Every time I opened a drawer with two unlabeled bottles inside, regret followed. It’s easy to forget what’s inside after a weekend, let alone six months. Mark the name, concentration, and the date it arrived. Permanent marker rarely wipes off until you need it to.
Why It Matters
Improper storage means risk. N-(2-Ethoxyethyl)-pyridinium bromide, like many pyridinium compounds, might irritate your skin or mucous membranes. Long-term, the bromide ion can corrode certain metals or cause container damage. Missteps invite spills, leading to cleanup costs and possible chemical burns. Using up budget and time on replacement bottles because of a simple oversight does not make anyone look good at work.
Following manufacturer recommendations reduces waste and headache. If you haven’t read the safety data sheet on your chemical’s first day in the lab, take a few minutes. It usually spells out if a chemical wants refrigeration, a desiccator, or its own special cabinet. Failing to do this once led to a chalky mess nobody wanted to clean a year later.
Common Sense Goes a Long Way
Don’t store incompatible chemicals together. Acids and bases may cause reactions, organic solvents near open flames invite disaster, and some oxidizers need more distance from everything. We always kept a printed chemical compatibility chart taped inside the supply room door. Simple steps tend to save a lot of trouble later on.
Protective gloves and goggles aren’t just for handling. Cleaning up a spilled bottle of N-(2-Ethoxyethyl)-pyridinium bromide with bare hands risks skin irritation and chemical exposure. Easy routines—label, seal, store smart, check inventory—make the chemical world a little less hazardous for everyone.
Chemicals Come with Questions
Every time a new chemical shows up in the lab or industry, safety usually becomes the first concern. N-(2-Ethoxyethyl)-pyridinium bromide isn’t a household name. Most folks will never handle it, but chemists, researchers, and workers in specialty manufacturing might. People deserve straight answers about what they’re working with and if it’s safe to breathe, touch, or store in their workspace.
What Happens on Contact?
Pyridinium compounds, as a group, bring their own set of hazards. N-(2-Ethoxyethyl)-pyridinium bromide contains a pyridine ring—which releases strong odors and causes irritation when inhaled. That ethoxyethyl arm ups the ante—solvents based on ethoxyethyl structures can sneak through skin or cause problems if mishandled. With bromide in the mix, there’s a chance of greater reactivity or sensitization. Companies that make safety sheets for this compound warn about skin and eye irritation, possible breathing issues if dust or vapor escapes, and environmental risk if it enters the water system.
Research, Regulation, Real-life Impact
Digging into scientific studies helps people see potential hazards. Reports on similar pyridinium salts show effects such as nausea, dizziness, or headaches. Toxicology tests point toward cell damage at higher concentrations. I’ve seen coworkers in research settings develop mild allergic symptoms after unprotected handling of related compounds.
Right now, N-(2-Ethoxyethyl)-pyridinium bromide doesn’t pop up on major restricted lists like California’s Prop 65 or the EU’s REACH with a ban label. That gives a sense that with proper safety procedures, the risks stay manageable. Truth is, many chemical companies emphasize “handle with care” because too little data exists on long-term exposure.
Chemical Safety Demands Respect
Hazardous doesn’t always mean deadly at a drop, but experience taught me to never underestimate a compound I don’t fully know. Once, during a university internship, I brushed off a minor skin rash after working with a new chemical—only to learn the next week that it could cause sensitization in some people. From then on, gloves and goggles became non-negotiable.
N-(2-Ethoxyethyl)-pyridinium bromide responds well to basic lab precautions. Good ventilation, gloves, safety glasses, lab coats—those are the unsung heroes. I’ve spent years in labs where the strictest teams rarely had incidents because they respected every label, even those lacking detailed hazard studies.
Protecting People and the Planet
Most chemical exposures happen not through dramatic accidents but through routine, low-level contact—think powder on hands, vapors in closed rooms, or spilled residue lingering on glassware. Better training and frequent safety checks stop the sort of slow-burn exposures that add up over time. I’ve seen labs cut risks sharply just by running short training sessions and posting straightforward reminder signs about PPE and spills.
Waste disposal makes another difference. Pouring this compound down the drain isn’t just bad form—it could introduce toxic bromide or pyridine byproducts into the ecosystem. Most labs collect and ship even small amounts of waste for proper treatment. That keeps water and soil cleaner, and my own decision to follow strict disposal protocols came after a local incident where improper chemical disposal led to expensive clean-ups and long-term soil checks.
Moving Forward with Knowledge
No one needs to panic, but a little healthy caution around N-(2-Ethoxyethyl)-pyridinium bromide goes a long way. Building a culture where questions about chemical safety get real answers—and doing the basics right—protects not just workers but the larger community. Trust in tried-and-true safety gear, thorough disposal plans, and transparent risk communication serves everyone better than guesswork.
Understanding the Role of Purity
Purity makes or breaks performance in chemical research. I’ve spent years around labs, and the frustration of dealing with off-spec compounds still stings. When researchers or manufacturers purchase N-(2-Ethoxyethyl)-Pyridinium Bromide, they expect a material meeting tight purity standards, usually 98% or greater. This benchmark sits above the threshold for most analytical and synthetic processes. Chemists depend on this consistency, as even a percent or two of unknowns can throw off a reaction, disrupt expected yields, or inject safety concerns into the lab.
What Specifications Matter
Start with the Certificate of Analysis; it holds most of the answers. A reputable supplier lists purity (often by HPLC), melting point range, and moisture content. Purity by HPLC confirms chemical integrity. Moisture analysis ensures the salt hasn’t picked up water along the way, which can affect both handling and reactivity. For N-(2-Ethoxyethyl)-Pyridinium Bromide, buyers often look for a pale powder with a melting point close to the literature value. Small changes here can flag contamination or degradation. The supplier should include trace metal analysis, making sure heavy metals remain below regulatory limits.
Supplier Reliability and Traceability
Not all chemicals are born equal. Over the years, I’ve seen how a robust supply chain makes all the difference. A trusted supplier shares batch data, outlines storage conditions, and provides a recent analysis. These steps matter for traceability and for building trust with clients who rely on predictable outcomes. Investors, auditors, and lab managers all breathe easier when every bottle traces back to a verifiable production run with defined specs. High-impact research and industrial batchwork lean on that reliability.
The Importance of Transparency in Manufacturing
Transparency isn’t just a buzzword here. In my experience, labs have faced real problems buying from vendors who won’t share full analytical reports or who omit impurity profiles. Worse, non-compliance with REACH or GHS labeling results in legal headaches, especially for institutions with strict procurement rules. Timely, accurate documentation keeps teams compliant, especially when international shipping crosses regulatory lines. Labs can’t afford to lose precious time double-checking paperwork or disputing quality claims after a shipment arrives.
Challenges and Solutions for End Users
Imperfect material costs more than just money—it leads to failed experiments, wasted time, and safety risks. Open lines with a vendor help catch problems early. Researchers and procurement departments need to ask for recent CoAs, traceability details, and full certification with every order. Independent testing builds an extra layer of protection, verifying that what arrives matches what’s on paper. This becomes especially important for high-value or large-quantity chemical orders where a single misstep means hours—or days—lost to troubleshooting.
Strengthening Best Practices
I always advise teams to document every batch, note storage temperatures, and catalog any variance in appearance or behavior. These habits don’t just support better science—they simplify recalls and safety assessments if something goes wrong. Teaching new lab members to look past the label and demand deeper information from vendors fosters a culture of vigilance that pays off in safer, stronger results. As N-(2-Ethoxyethyl)-Pyridinium Bromide supports applications from catalysts to pharmaceuticals, these fundamentals ensure innovation happens on solid ground.