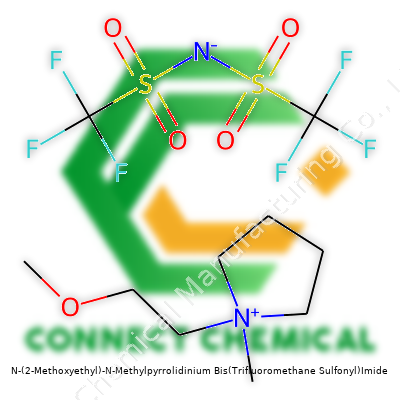
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bis(Trifluoromethane Sulfonyl)Imide: A Deep Dive
Historical Development
Long before the world sensed the weight of advanced electrolytes, scientists searched for alternatives that could perform under heat and stress. The 1990s saw a surge in ionic liquid research. Chemists looked past traditional salts, moving their gaze to compounds that delivered both stability and unique field characteristics—an urge driven by battery, synthesis, and separation demands. From those ideas, the pairing of pyrrolidinium cations and bis(trifluoromethanesulfonyl)imide anions emerged. This class, built around imide anions, gave the world N-(2-Methoxyethyl)-N-Methylpyrrolidinium bis(trifluoromethanesulfonyl)imide, widely dubbed [MEMPyrr][TFSI] in specialist circles. Over years, developers saw that not all ionic liquids behaved the same. Small tweaks, like adding a methoxy group to the alkyl chain, paved the way for fine-tuned physical and chemical traits. Today, this compound stands out for its use in batteries, electrochemistry, and beyond.
Product Overview
People who’ve worked around high-performance chemicals spot [MEMPyrr][TFSI] thanks to its clear liquid look and signature faint odor. It doesn’t attract water from the air like some of its cousins, so moisture control during handling still matters. Production often centers in specialty chemical labs, with purity running above 99%. Formulators demand exceptional quality for those working in critical electronics and research. Due to its key role in sensitive environments, most suppliers package it in amber glass bottles or specialty polymers, taking care to mark clear hazard and handling protocols on each shipment.
Physical & Chemical Properties
Years of data show this ionic liquid remains stable up to about 350°C, resisting breakdown in most industrial settings. Its viscosity sits in the intermediate range—not as sticky as the most sluggish salts, not as runny as solvents like acetonitrile. The liquid’s density hovers slightly above that of water, and its broad electrochemical window—stretching roughly from -2.5V to +2.5V—makes it a popular pick for battery developers and electrochemical researchers. Conductivity runs strong, especially when the humidity drops off, proving valuable in all sorts of in-vivo and high-voltage environments. The low melting point (usually below -20°C) gives flexibility in temperature-critical work. From experience in the lab, the slight ether-like note—the signature of the methoxy group—alerts experienced noses, but isn’t overpowering or hazardous without serious overexposure.
Technical Specifications & Labeling
On each bottle, you’ll spot the chemical’s CAS number, structural diagram, and concentration. Purity often lists at over 99%, with trace moisture kept below 100 ppm. Most reputable producers include a batch certificate, breaking down NMR, GC-MS, and Karl Fischer titration results. You’ll find hazard codes for skin and eye sensitivity, as well as storage instructions: keep away from direct sunlight and oxidizers, stabilize between 15°C and 25°C. All labeling follows globally harmonized system (GHS) rules, with pictograms and QR codes linking to safety data sheets for quick digital access.
Preparation Method
From bench-scale work, building [MEMPyrr][TFSI] starts with N-methylpyrrolidine, which reacts with 2-methoxyethyl chloride to form the quaternary ammonium cation through a nucleophilic substitution. Batch reactors keep anhydrous conditions, as excess water introduces byproducts that sap downstream yield. The resulting N-(2-methoxyethyl)-N-methylpyrrolidinium chloride then meets lithium bis(trifluoromethanesulfonyl)imide in a one-pot exchange. This salt metathesis liberates lithium chloride and leaves the ionic liquid behind. After extraction with dichloromethane or similar solvents, technicians dry the product under reduced pressure to avoid hydrolysis. Laboratory experience tells that solid-phase extraction cuts down water traces in the end material, which matters for anyone aiming for precision.
Chemical Reactions & Modifications
Several folks in research circles play with [MEMPyrr][TFSI] as both a solvent and ionic medium. The ether side chain lets more lithiophilic interactions, so in some battery setups, the ionic liquid can dissolve lithium salts better than simpler pyrrolidiniums. The cation withstands alkali conditions, but strong acids break it down, cleaving the ether link. Chemists sometimes swap out the methoxyethyl group for other substituents aiming for different conductivity or solubility, yet the foundation remains the pyrrolidinium backbone. The anion—TFSI—offers broad resistance to oxidative attack, yet reacts with certain nucleophiles and strong reducing agents.
Synonyms & Product Names
Depending on supplier or literature source, you’ll see names like N-(2-methoxyethyl)-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)imide, MEMPyrr TFSI, or less formally, Pyr13O1 TFSI. In shorthand, ionic liquids enthusiasts call it [MEMPyrr][TFSI]; salts packaging sometimes bears the numbers 428837-64-7, tying it to global chemical catalogs. For the sake of safe shipping, many companies adopt code-based trade names, hiding detailed structure in proprietary documentation sent ahead to qualified buyers.
Safety & Operational Standards
People working around [MEMPyrr][TFSI] learn the routines fast: gloves, goggles, dedicated bench space. Contact with skin leaves an oily film but seldom burns, unless there’s a hidden cut or abrasion. At higher concentrations, the vapor isn’t pleasant, but real risks come if anyone ingests or injects it, or lets it build up in an unventilated room. Disposal remains guided by local chemical safety rules—incineration at licensed plants with proper capture for fluorinated byproducts. Regular users inspect for equipment corrosion, especially in reactors with exposed metals. Industry standards mandate annual safety reviews, incident reporting, and frequent refresher training to keep labs in line with international best practices.
Application Area
From my experience running test batteries and prepping electrochemical sensors, this ionic liquid fits in electrolyte blends chasing better cycle life and broader voltage spans. It steadies the charge transport in lithium-ion batteries, especially for high-voltage chemistries that chew through fragile solvents. In capacitors, it delivers the kind of low resistance needed for high power work. Researchers like its low volatility—so devices keep working even when temperatures swing. Analysts use it to pull apart tough-to-separate organic mixtures in chromatography—its unique chemistry unearths patterns regular solvents miss. Supercapacitor teams see value in both the stability and compliance with emerging environmental rules banning older, toxic phthalates.
Research & Development
Collaborations in university and industrial labs stay strong in this area. Researchers run studies testing [MEMPyrr][TFSI] against newer ionic liquids, studying factors like ion mobility, viscosity at different scales, and performance in real-world devices. Funding agencies target projects on post-lithium batteries and green process chemistry, making stable, non-volatile media like this a focus. In my time around battery pilot lines, teams spent months cross-checking results with and without specialized additives—building up a knowledge base that now feeds shared patent filings and third-party data reviews. Proprietary blends, guided by trial outcomes, continue to shape future device performance and cost stability.
Toxicity Research
Groups studying toxicity take a careful, methodical approach. Early data reflected minor irritation if splashed onto skin, but chronic effects required longer trials. So far, animal studies show low bioaccumulation, yet liver metabolism of fluorinated residues keeps toxicologists vigilant. Regulators look for traces in wastewater streams, and some exploratory work tests whether plant life near disposal sites absorbs breakdown products. Cornell and MIT groups published findings showing minimal air volatility, lowering inhalation worries for those outside active labs. Risk profiles see constant updating, especially with new publication cycles or shifts in environmental policy.
Future Prospects
With battery demand booming, especially for electric vehicles and grid storage, chemists and engineers forecast a continuing climb for [MEMPyrr][TFSI]. Ongoing development targets lower manufacturing costs and expansion to sodium- and potassium-based battery systems. Calls for safer lab and manufacturing environments push further testing—teams want to make sure next-gen devices built with ionic liquids meet all local and global safety rules. A shift toward biocompatible ionic liquids spurs new cyclization and modification projects. Researchers rally to crack the old barriers in scalability and cost, aiming for broad adoption in applications as diverse as antistatic coatings and novel catalysis. Thanks to years of careful study and industry feedback, the compound holds ground in academic journals and patents, with every sign it will shape the next leaps in clean tech and advanced materials.
Why This Compound Gets Attention
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bis(Trifluoromethane Sulfonyl)Imide, often called PYR13TFSI, sounds like a mouthful, but it’s not just lab jargon. Most folks working with advanced materials or batteries have bumped into it. This ionic liquid doesn’t boil off like many organic solvents, shrugs off water, and holds up under a current. Through my years tinkering with battery labs and cleanrooms, I’ve seen how game-changing these properties turn out to be.
Building Better Batteries
Lithium-ion batteries make the world move, literally. Phones, electric cars, even massive grid storage—they all count on safer, stronger electrolytes. PYR13TFSI steps up because it doesn’t burst into flames if the cell malfunctions. In my early days in battery prototyping, watching an old-school solvent catch fire during stress tests hammered home the value of safer chemical choices. Manufacturers favor this ionic liquid for next-generation lithium and sodium batteries because of its low volatility and wide electrochemical window. The salt menagerie inside these fluids lets fast-charging and long-lifetime chemistries unfold, standing up to the abuses of real life—hot, cold, jolts, and all.
Greener Chemistry and Industry
Toxic solvents wreck more than lab coats—they leak into the air and water. Regulatory bans keep getting tighter. PYR13TFSI sidesteps much of that mess. It doesn’t release much vapor, even at high temperatures, so chemical plants and research labs use it for tough reactions like alkylations, organic syntheses, and specialized separations. For anyone with allergies or concern for environmental damage, being able to use a liquid that’s less likely to wind up as air pollution or groundwater run-off is a relief.
Supercapacitors and Energy Devices
The energy world doesn’t just revolve around batteries. Supercapacitors offer something close to instant energy delivery—a quick burst for trains or grid balancing. They need ionic fluids that don’t freeze in the cold or decompose when current flows thick and fast. I’ve seen teams skirt around clunkier salts and classic organic solvents because PYR13TFSI soaks right into porous carbon electrodes without degrading. The high thermal stability also means supercapacitors can sit on the shelf or under the hood longer than most alternatives.
Electroplating, Sensors, and Niche Roles
Metal finishing buffs and researchers find value here. Electroplating fancy alloys or rare metals gets tricky with water-based systems full of side reactions. PYR13TFSI’s broad stability window lets operators lay down thin films of specialty metals—aluminum, rare earths—where water would sputter out. Over the last few years, the sensor world has started picking up on ionic liquids for biosensors, environmental sensors, and exotic conductive inks. Durability and low volatility keep the readings reliable longer, especially in harsh environments.
Room for Growth
The story isn’t all solved. The cost of making ionic liquids, especially those with long, complex names, still runs high. Labs and manufacturers keep looking for ways to scale down price and scale up sustainability without ceding performance. It’s a common concern: balancing cost, safety, and performance. Anyone who’s wrestled with a grant budget or plant invoice knows this dance. Still, with more folks investing in cleaner and more robust chemistries, PYR13TFSI looks set to show up across bigger slices of industry and technology. Keeping an eye on purification, greener synthesis, and end-of-life recovery will help make these applications stick in both the lab and the real world.
Day-to-Day Frustrations Spark Lessons in Storage
I once found a forgotten bottle of a reagent stashed in the back of a shared lab fridge. The label had faded, the cap had cracked, and nobody could remember who brought it in. Everyone shrugged, assuming chemicals don’t change—after all, the powder looked fine. A rookie move. Months later, that same bottle triggered problems in an experiment, forcing a retest. Stories like this pop up all over research, from academic labs to major manufacturing facilities. The truth is: chemical stability isn’t just about shelf life. It touches safety, budget, and all those little details that keep projects on track.
The Science Matters: Heat, Light, and Even That Cap
Moisture sneaks in through loose seals and can turn certain powders into sludge or trigger slow, silent reactions. Organic peroxides love to break down under UV light—one ray and you’re left with something useless or even hazardous. Oxygen can turn sensitive compounds into a chemical soup. Then take temperature: a fluctuation of just a few degrees can start the decay of pharmaceuticals or specialty reagents. Heavy metals sometimes catalyze unwanted reactions; even glassware can leach ions—those who’ve lost a batch to bad bottles know the pain. Every bottle needs a home, whether it’s a dry cabinet, the cold shadow of a freezer, or the amber glass that shields light-sensitive wonders.
Why Labels, Logs, and Audits Beat Wishful Thinking
Chemical stability shouldn’t be a guessing game. Permanent markers and waterproof labels save hours of backtracking, especially if someone stores two clear liquids side by side. I learned early to keep a storage log, jotting down purchase and opening dates, storage moves, and even odd smells when something seemed off. This habit paid off. Once, a stable custodian caught a volatile acid stored upright instead of tightly crimped under nitrogen. The early catch stopped a catastrophe and a thousand-dollar loss. Trust in protocols—safety and cost tracking go hand in hand.
Solutions for Lab and Field—With Facts
Dry boxes powered by desiccants handle moisture-prone reagents. A recent CRC review flagged that certain pharmaceuticals degrade ten times faster at 25°C than at 4°C, making temperature logs worth every penny. VWR and Fisher Scientific carry light-resistant bottles that keep photosensitive compounds intact. I’ve switched over to these for any chemical with a NIST sheet warning of photolability. Adding antioxidants, or using inert gas blanketting, makes sense for oxygen-sensitive materials—cost upfront saves batches later.
The old habit of shoving everything on the same shelf disappears after one contamination scare. Color-coded storage bins, daily fridge checks, and digital inventory apps keep dangerous combinations apart. Labs using risk assessments before introducing new chemicals keep incidents low. Over the years, I’ve watched slack habits cost companies thousands in wasted stock and ruined experiments.
People Make Stability Work
No single fix solves every challenge. Experience grows through trial and error, swapping notes with colleagues, and keeping up with regulatory bulletins. The best setups involve constant checks, open communication, and a respect for details. Seasoned chemists and techs look at each vial as part of a living system—one small oversight can ripple into big setbacks. Smart storage and stability planning set a foundation for progress, safety, and clean science.
Looking Past the Hype
Ionic liquids get plenty of attention as “green” solvents. People talk about how these liquids don’t vaporize like the old-school organics. No dangerous fumes, no fire risk. That sounds good, but people sometimes stop the conversation there. In my years working with these substances in both chemical research labs and pilot plant settings, I learned that the full safety story needs a deeper look.
Toxicity Depends on the Structure
Ionic liquids aren't a single thing. You’ve got hundreds of options by tweaking the cation and anion. That means toxicity is all over the map. Some early publications painted them as almost harmless compared to traditional solvents—especially the ones based on imidazolium. Now, updated studies show some of those same structures can do real damage to aquatic life. Diluting them with water doesn’t solve the issue if the compound doesn't break down.
From experience, skin contact causes a strange tingling sensation with certain kinds. I learned to keep gloves on and check glove compatibility with specific ionic liquid salts before pouring or filtering. That physical warning matters: a slick green reputation doesn’t protect your skin or lungs if you get careless.
Environmental Impact Can't Get Ignored
While folks call ionic liquids “green” because they don’t evaporate, that only tells us part of the story. Persistence in water is a huge concern. The more stable these molecules are, the longer they stick around in wastewater or the environment. I’ve seen university labs struggle to safely dispose of them; our group often had to collect ionic liquid waste separately, just to keep it out of drains. Research shows some ionic liquids resist natural degradation, and chronic low-level exposure can upset the balance in microbial communities.
Handling ionic liquids outside regulated environments always made me uneasy. If someone just washes a beaker down the sink, there’s no easy fix after that. Responsible groups now push for “benign by design”—crafting liquids to break down quickly after use. More manufacturers are starting to label products with ecological and toxicity data, but the field lags behind for less common salts.
Simple Steps Anyone Can Take
Fume hoods make a big difference. Even without high vapor pressure, splashes or accidental drops release small particles you can inhale or get on skin. I always wore nitrile gloves and goggles, and kept an emergency eye-wash station close, just in case. New trainees get drilled on how to clean up spills, since certain ionic liquids stain skin quickly and stick to bench tops much longer than water or ethanol.
Product safety data sheets vary in quality. Some list full toxicology reports; some only say “not fully tested.” If the data stops at “not hazardous,” ask for more—especially if the liquid carries uncommon cations or fluorinated components. Reading academic articles on known structures helped me judge risk better than trusting a supplier’s catalog.
What Researchers Suggest
No one wants to slow research down, but people need to factor in persistence, toxicity to aquatic life, and risk of exposure when picking these solvents. Choosing ionic liquids with simple, biodegradable ions can help. Industry and public groups now back projects that study long-term breakdown and safe disposal routes. A few companies invest in closed-loop systems to collect and recycle used ionic liquids, cutting down both cost and environmental risk.
Being cautious pays off. If something feels off—a strong odor, irritation, trouble cleaning a spill up—find support, check the literature, and switch to a safer option if possible. That keeps both the lab team and the folks downstream safer in the long run.
Understanding Everyday Materials
Ever tried to cook with butter on a hot day and found it melted before it hit the pan? Or mixed sugar into your morning coffee and noticed how quickly it disappeared? These ordinary moments showcase two of the most practical physical properties—melting point and solubility. They work behind the scenes, shaping experiences not only in the kitchen but everywhere materials get used, mixed, or changed.
Melting Point: A Window into Stability
Melting point counts as a material’s calling card. Defined as the temperature where a solid moves into a liquid state, this property has practical meaning. Take ice, melting at 0°C (32°F). Drop some cubes into a summer drink, and you see it: they hold their shape until heat wins out, and then in moments, they vanish into water.
I remember working in a cramped college chemistry lab, where handling different solids always called for attention. Substances with low melting points, like wax or certain plastics, could warp and run with just the heat of your hand. Metals such as iron, boasting high melting points, sat stubbornly solid despite high flames. These differences create real-world safety boundaries for workers handling hot liquids, foundry castings, or industrial coatings.
Melting point does more than determine whether a substance liquefies on a hot sidewalk. It acts as an early quality checkpoint in manufacturing and pharmaceutical development. Tire companies use rubber blends precisely adjusted to withstand scorching highways. Drug makers test melting ranges closely because impurities can shift this value, hinting that something unwanted creeped in.
Solubility: From Drinks to Doses
Solubility describes how well a substance mixes into another—common wisdom most folks know instinctively. Toss table salt into water, and it quickly vanishes. This property might not grab headlines, but it quietly decides how things work in health, cleaning products, and environmental fixes.
Teaching high school chemistry, I ran into plenty of puzzled looks when we added oil to vinegar. No matter the stirring, those liquids would never mix. On the other hand, a packet of powdered drink mix dissolves beautifully in water, delivering color and taste in every sip. Such observations reveal more than kitchen curiosity—they shape how medications reach the bloodstream or pollutants spread in groundwater.
Think about pharmaceuticals. A pill that dissolves poorly in the stomach doesn’t deliver results. Researchers shape molecules with this in mind. Vitamin C dissolves easily, but certain vitamins, like D and E, need fat for absorption. This drives the design of supplements and guides dietary suggestions.
Working Toward Better Outcomes
Everyday challenges can hinge on melting point and solubility. Imagine a road crew needing deicers that keep roads safe without harming the landscape—properties like melting point decide which chemicals to choose, while solubility charts the cleanup story when rain comes. Chemists search for better options all the time, aiming to lower risks and improve performance.
Tapping into these properties means running honest, hands-on tests and not relying on guesswork. It takes careful measurements, solid training, and a willingness to try new combinations. Technical skill meets common sense at this intersection. Every time you mix your coffee or grab an ice pack, you’re experiencing these principles yourself—making science part of daily life in quiet but powerful ways.
Understanding the Chemical’s Market
Anybody who has spent time in a lab or managed a procurement line for research chemicals knows how much effort goes into sourcing specialty compounds. N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bis(Trifluoromethane Sulfonyl)Imide, often called PYR14 TFSI, pops up in energy storage research and ionic liquids development, with labs using it for battery electrolytes or as a neat solvent in synthesis. Here’s what matters: not every supplier stocks this one due to its niche status and price.
Reputable Sources and Safety
Chemists stick to trusted chemical suppliers—Sigma-Aldrich, Alfa Aesar, TCI, and Fisher Scientific. These companies require documentation, account setup, and verification of intended use. It’s not paranoia. This process keeps both the buyer and the seller within the boundaries set by safety and regulatory authorities. Safety data sheets (SDS) accompany every order and spell out the risks in plain terms. Having spent hours reviewing these sheets, I can tell you not every chemical is created equal when it comes to hazard. Ensuring purity and storage conditions matters, since a subpar sample can spoil experiments and, worse, cause accidents.
International Considerations
Ordering across borders creates extra layers of paperwork. Importers run into customs checks, special licenses, and environmental scrutiny, especially in Europe and North America. For some countries, navigating this can eat up more time than the research itself. Blindly ordering from websites promising overnight shipping can bring customs headaches and sometimes run you afoul of local law. That’s a lesson anyone working with less common solvents learns quickly.
Online Marketplaces and Risks
A few sites list this compound but don’t display price or check credentials up front. Some even skip technical support, which makes troubleshooting impossible if something goes wrong with your batch. That sort of gamble rarely pays off. I remember waiting weeks for a specialty reagent from a lesser-known seller, only to discover the bottle arrived poorly sealed. Loss of material and wasted time never look good on a project’s record.
Smart Sourcing: Tips From the Lab Bench
Experienced buyers reach out directly to the sales teams at big chemical suppliers, especially for high-purity or large-volume orders. That opens doors for technical guidance. Many suppliers offer certificates of analysis (CoA) and even run batch-specific impurity checks on request. For organizations ordering for the first time, companies like Merck or Alfa Aesar ask for institutional emails and signed usage declarations. Some countries also require end-user agreements to prevent diversion into the wrong hands.
Alternatives and Substitutes
If budgets run tight or lead times stretch out, some researchers shift to related ionic liquids. This change impacts experimental results, so nobody takes it lightly. It always helps to ask larger universities or companies if they have a stock they’re willing to share. Trading small quantities among established labs sometimes plugs gaps, but these exchanges require transparency on both sides, particularly regarding purity and storage.
Final Practical Advice
Clear communication gets results. Clarifying purity requirements and volume speeds things up. Detailed documentation keeps everyone covered during inspections or audits. Picking a supplier with a track record for both safe handling and regulatory compliance gives peace of mind, not just for your project but for everyone working downwind of that chemical cabinet.