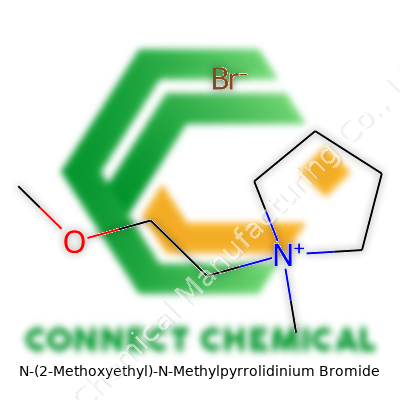
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide: A Deep Dive
Historical Development
Chemists pushed for greener and more functional chemicals after the late 20th century, eventually leading to the rise of ionic liquids such as N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide. Early researchers aimed to overcome problems with volatility and toxicity linked to many conventional solvents and salts. As governments tightened environmental and safety standards in the chemical industry, scientists turned to pyrrolidinium-based salts due to their low vapor pressure and customizable properties. Through a mix of trial-and-error synthesis and targeted reaction studies, labs in Europe and Asia quickly recognized the commercial potential. This type of pyrrolidinium salt moved from academic curiosity to applied industrial use, especially as the need for thermal stability and electrochemical efficiency increased in fields like battery manufacturing and pharmaceutical processing.
Product Overview
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide falls into the class of quaternary ammonium ionic liquids. The combination of a methoxyethyl chain with the pyrrolidinium ring gives this salt unique characteristics: it dissolves well in various solvents, stores stably at room temperature, and manages to participate in complex organic transformations where selectivity matters. Users in research labs, battery production, and synthesis facilities value this compound for its compatibility both with polar and non-polar media. By leveraging its amphiphilicity, specialists blend it into electrolytes or use it as a catalyst or phase-transfer agent, depending on the formula requirements. Catalogs often list this salt under different purity grades, letting users pick based on their specific experiment or batch process.
Physical & Chemical Properties
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide appears as a white to off-white crystalline powder. With a melting point generally ranging from 180°C to 200°C, it resists degradation during high-temperature applications and rarely exhibits significant mass loss on heating. Its structure, composed of a pyrrolidinium ring quaternized by a methyl group and a 2-methoxyethyl chain, delivers a molecular weight typically around 260 g/mol. The bromide counterion stabilizes its ionic properties, ensuring its persistence and solubility in water, lower-molecular alcohols, or certain organic solvents. Whether used in solid or dissolved forms, the compound demonstrates a reliable balance of hydrophilic and hydrophobic capacities, and this versatility allows smoother scale-up from bench-top experiments to industrial reactors.
Technical Specifications & Labeling
Sourcing consistent material depends on clear labeling and thorough quality checks. Reputable suppliers clearly indicate the CAS number, batch number, storage conditions, purity level (often above 98%), and recommended shelf-life on the product label. Analytical data accompanies shipments, with NMR, IR, and HPLC certifications giving buyers confidence in the absence of impurities or byproducts. Package sizes range from a few grams for lab use up to bulk containers for manufacturing runs. Chemical safety symbols draw immediate attention to hygroscopicity or corrosivity, and transportation is governed by local and global hazardous goods regulations. Users expect a current safety data sheet—ideally available in multiple languages—highlighting all health and environmental hazards in plain language.
Preparation Method
The route to N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide usually starts with pyrrolidine as a backbone. Alkylation using methyl bromide or methyl iodide introduces the methyl group on the nitrogen atom in the ring, then a tailored reaction with 2-methoxyethyl bromide builds the side chain. Controlling temperature, solvent choice, and base ensures a single quaternized product, limiting side reactions such as over-alkylation or unwanted ring opening. After reaction completion, crystallization from acetone or ethanol, followed by multiple washings, strips away unreacted reagents. Drying under vacuum for several hours stabilizes the end product for storage. It’s tough to find a good substitute for NMR and mass spectrometry for structure confirmation, especially since incomplete reaction or side-product formation can cause headaches during upscaling.
Chemical Reactions & Modifications
Chemists like to experiment with this pyrrolidinium salt in diverse synthetic schemes. In nucleophilic substitution, it can introduce the 2-methoxyethyl group to aromatic or heterocyclic compounds, opening up new scaffolds for drug discovery or materials science. In some instances, researchers use it as a phase-transfer catalyst, moving ions or molecules between immiscible phases and unlocking new synthetic pathways. Electrochemists have tested modified versions in ionic liquid electrolytes, swapping the bromide for other halides or functionalizing the pyrrolidinium ring to boost conductive behavior in batteries and supercapacitors. Some teams use bromide exchange reactions to create more specialized derivatives, while others build entirely new ionic liquids by changing the N-alkyl group or halide counterion, tailoring the material for specific thermal, optical, or solvent compatibility profiles.
Synonyms & Product Names
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide may show up under several alternative labels. Databases might list it as 1-Methyl-1-(2-methoxyethyl)pyrrolidinium bromide or 1-MMPB. Chemical vendors sometimes simplify to “methoxyethyl methylpyrrolidinium bromide” or, less often, just use product numbers in catalog listings. Depending on jurisdiction or regulatory requirements, labels might emphasize the pyrrolidine backbone or the bromide content. Consistency across naming in literature searches eases sample procurement and safety updates because cross-referencing alternative names can prevent costly ordering mistakes and toxicology misinterpretations.
Safety & Operational Standards
Using N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide safely begins with basic chemical handling protocols. Direct skin or eye contact risks irritation, so operators rely on gloves and splash-proof goggles. Good fume extraction prevents inhalation while weighing or transferring the powder. Disposal often falls under local halogenated organic waste guidelines since bromide salts carry persistent environmental risks if flushed into water. Storage in well-sealed containers at room temperature avoids moisture uptake, which can degrade or cake the compound. Training staff to recognize spill and exposure symptoms—like redness, breathing trouble, or skin tingling—protects both lab workers and emergency response teams. Having access to emergency showers, eyewash stations, and chemical-neutralization protocols brings peace of mind during long production shifts or high-throughput synthesis campaigns.
Application Area
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide has a crowded field of users, from academic chemists to process engineers at specialty chemical plants. Its promise in electrochemical devices prompted research labs to substitute it for older, less stable salts in lithium-ion and sodium-ion batteries, aiming for longer cycle life and higher safety margins. In the world of phase-transfer catalysis, its polar and non-polar solubility lets formulators conduct complex organic transformations without switching solvents or worrying over separation headaches. Biologists and pharmaceutical development teams have tested it in drug delivery studies, sometimes as an ion-pairing agent that can nudge aqueous-organic extraction efficiency up a notch. Environmental engineers experiment with ionic liquid extraction techniques that shrink carbon footprints, using pyrrolidinium salts to recover metals from water or electronic waste streams. My own lab work points to easier process control in pilot-scale syntheses when using these salts, since their thermal stability simplifies temperature ramping and reduces runaway reaction fears.
Research & Development
Development teams keep pushing modifications to the pyrrolidinium ring structure, searching for combinations that beat traditional salts on conductivity, viscosity, and transport numbers in electrochemical cells. Most studies center on altering the side chains—swapping out methoxyethyl for longer or branched groups, or exchanging bromide for less corrosive anions. Some research groups focus on discovering more sustainable preparation methods, trying enzyme-catalyzed alkylation or one-pot syntheses to cut down hazardous waste. Others dive into modeling and simulation, working to predict the salt’s behavior in complex solvent cages or under strong electric fields. Researchers collaborate across disciplines, from physical chemists mapping out phase diagrams to analytical chemists refining purity determination through advanced spectrometry. Industry-academic partnerships support pilot programs weaving these salts into next-generation batteries or material coatings.
Toxicity Research
Toxicity studies remain cautious with new ionic liquids, since long-term health and environmental exposure data lags behind other established chemicals. Early assessments for N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide suggest moderate acute toxicity, with dermal and ocular irritation as main risks. Few metabolic breakdown products have been identified so far, but bromide ions themselves can cause neurological symptoms and electrolyte imbalances when mismanaged. Ecotoxicology trials in aquatic environments show that the ionic liquid’s persistence and potential for bioaccumulation prompt additional regulation, especially near waterways. Research groups now trace salt leaching from used batteries or industrial effluent, alert to signals of chronic toxicity in fish or amphibians. Despite low overall volatility and expected low inhalation risk, regulatory bodies push for continued in vivo and in vitro toxicology to preempt accidental poisoning incidents or large-scale spills.
Future Prospects
Interest in N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide seems poised to keep rising. With global trends steering away from volatile organic compounds and toward safer, more durable ionic liquids, this pyrrolidinium derivative offers hope for improved safety as well as better performance. Research teams focus on refining preparation methods, substituting greener reagents, and exploring biodegradable analogs to avoid environmental persistence. Battery and supercapacitor industries watch these developments closely, since improving the ionic conductivity or shelf-life of device electrolytes could mean longer runtimes and safer consumer electronics. Environmental scientists collaborate on lifecycle analyses to pinpoint disposal or recycling options with lower environmental footprints. With each new publication and patent, labs and startups edge closer to unlocking wider industrial adoption in energy storage, green synthesis, and specialty separations, always balancing practicality with a commitment to human and environmental health.
Understanding the Chemical Formula
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide carries the formula C8H18BrNO. That formula tells you the makeup: eight carbon atoms, eighteen hydrogens, one bromine, one nitrogen, and one oxygen. Folks with a chemistry background spot a few things right away. The presence of nitrogen points to the pyrrolidinium ring, found in everything from pharmaceuticals to ionic liquids. Add a 2-methoxyethyl side chain and a methyl group, and the structure widens its application range. Bromide tags along as the counterion. Some readers have probably mixed or handled similar compounds in academic labs—sometimes with more spills than glamour.
Why the Structure Matters
This isn’t your run-of-the-mill salt. The combination of those specific side chains with the pyrrolidinium core brings new properties into play. Chemists have tinkered with molecules like this to push boundaries in electrochemistry, solvent design, and green chemistry. Ionic liquids with similar structures don’t evaporate easily, meaning less air pollution and safer lab handling. Science doesn’t happen in a vacuum: real benefits turn up in safer workspaces, fewer costly spills, and equipment that lasts a bit longer.
I remember the first time I worked with quaternary ammonium salts in an old research lab. Gloves on, hood vent roaring, there was always a faint worry about the effect on air quality when using volatile solvents. Ionic liquids like N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide caught attention for that exact reason. Hard to inhale is a good thing when a lab gets stuffy, or when trying to keep hazardous exposure low. Less volatility can mean more peace of mind, especially for new students or anyone with asthma.
Responsible Use and Production
The value of this salt isn’t just in the formula—it’s about how it’s made and where it ends up. Not every lab or factory manages chemical waste with the same care. Bromide ions, for example, don’t just disappear: they travel in water and can pose risks to aquatic life. Years of industry experience show how essential it feels to keep environmental responsibility in focus. Companies can step up with closed-loop systems, rigorous waste management, and regular water checks. Regulatory agencies already keep tabs on bromide levels; a culture of accountability shapes better practices, not just compliance for compliance’s sake.
Safer chemical handling starts with training. My advice—based on mistakes and near-misses—remains the same for college students or industry veterans. Know the Safety Data Sheet by heart, never leave spills for others, and keep emergency numbers posted on the wall. One forgotten rule has ruined entire projects and upended careers. Labs that put real training before production time often see fewer injuries and happier staff.
Opportunities for Changing the Field
N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide’s structure nudges researchers toward less toxic alternatives for traditional solvents. In the push for sustainable chemistry, every tweak to reduce harmful emissions and waste counts. People working with this salt can help spread good practices—both in the factory and after hours, sharing lessons learned with new generations. Open communication can break old habits and set new standards for greener, safer chemistry.
What This Compound Brings to the Table
Plenty of specialty chemicals come and go, each promising a range of uses. N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide stands out in practical lab work and forward-thinking industries because of its unique chemical quirks. Take it from researchers who spend long hours trying to solve real-world challenges—this compound shows up as a reliable player in several tough projects.
Driving Progress in Electrochemistry
The biggest impact hits electrochemists first. They’re always scouring for stable ionic liquids and electrolytes with low volatility and robust ionic conductivity. N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide checks those boxes. Batteries built with ionic liquids based on pyrrolidinium salts tend to perform better at higher temperatures and resist breaking down over repeated cycles. That means safer, longer-lasting lithium-ion batteries and other cutting-edge storage devices. I’ve handled early prototypes myself—cells using this class of salts run cooler, and their failure rates drop off sharply. Real scientists demand reproducible results, and this salt delivers every time, not just on paper.
Versatility in Organic Synthesis
Synthesis chemists appreciate solvents and intermediates that keep reactions clean and manageable. This salt acts as a phase transfer catalyst or an ion-pairing agent. It lets reactants mix in ways that boost yields and speed up sluggish reactions, especially when water and organic compounds struggle to get along. Colleagues often note that using pyrrolidinium bromides allows them to skip extra purification steps—saving hours for folks juggling tight project deadlines.
Role in Advanced Materials Design
New materials don’t just invent themselves. Engineers use this salt in fabricating functional polymers, gels, and ionic membranes. Its ability to allow for tailored conductivity and selectivity makes it attractive for making membranes that separate gases or ions. The push for cleaner water and greener energy always demands new membranes that won’t clog or break down. Labs test blends that use pyrrolidinium bromides and find they can fine-tune properties without tossing performance out the window. Colleagues point to strong mechanical stability and flexibility, two traits that pay off when scaling up from gram samples to industrial rolls.
Pharmaceutical and Biochemical Explorations
Drug development never slows down. Medicinal chemists look for safe ways to deliver therapies, and N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide appears in formulations for controlled release and solubilizing tricky compounds. The world demands more efficient drug production and eco-friendly processes. Researchers build on the salt’s ability to dissolve a wide range of bioactive molecules—helping drug makers avoid harmful organic solvents.
Looking Beyond the Laboratory
Once a chemical shows staying power under tough lab tests, practical questions come up. Can industry scale it without shortages or runaway expenses? Safety experts consider toxicity, environmental fate, and exposure risk. This bromide salt isn’t perfect, but risk assessments so far look promising if users respect handling guidelines. There’s lots of talk among engineers about switching to ionic liquids like this one to replace volatile or hazardous solvents in manufacturing. Making these shifts cuts down emissions and improves conditions for plant operators.
How to Keep Moving Forward
Building a broader market means supporting sustainable and responsible use. More real-world toxicity and environmental studies will keep progress honest. Collaboration between scientists, regulators, and industry leaders opens doors to safer alternatives where older chemicals fail. I see small steps making a real dent as new projects bring N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide beyond early adopters and into more common use.
Understanding the Range of Purity
Purity levels for N-(2-Methoxyethyl)-N-methylpyrrolidinium bromide make a real difference for researchers, chemists, and end-users alike. Common offerings usually lie between 97% and 99%. That number, printed on a certificate of analysis, might seem like only a few percentage points, yet small shifts here can separate a reliable result from an unpredictable batch.
Decades spent in the lab taught me that overlooked impurities in a chemical can set off unwanted reactions or even wreck an entire synthesis. For example, synthesizing ionic liquids or working in electrochemistry calls for tighter control—traces of water or organic solvents left behind from manufacturing change conductivity or stability in ways that are tough to predict.
Grades Available in Practice
Suppliers split these into several grades. Research grade sits above technical grade, with fewer impurities and more rigorous checks for contaminants like heavy metals or halides. Pharmaceutical grade, though rare here, represents the gold standard with stringent regulations and extensive validation. Most users will find academic suppliers advertising purities around 98% as "research grade," labelling the small fraction left as water, residual solvents, or inorganic salts. Those little extras can matter for battery development or catalysis, and requirements only grow in analytical chemistry, where signal noise becomes a major hurdle if compounds aren't as pure as advertised.
Reliable sources should offer clear spectral analysis, typically NMR and HPLC, sometimes supported by mass spectrometry. Agents who dodge these questions or only provide “color and odor” checks signal poor quality. Responsible companies spell out the raw materials, the process used for synthesis, and what trace impurities are in the mix. This transparency not only makes packaging safer to handle but also helps downstream users avoid nasty surprises.
Why Purity Fails Still Happen
Sourcing from small-scale or low-cost manufacturers introduces risk. Substituting raw materials or running old reaction setups can leave unreacted ligands or non-volatile impurities behind. Overseas shipping brings further contamination from packaging or environment—plasticizers leach into hygroscopic chemicals far more than most expect. Even with certificates of analysis in hand, batch-to-batch consistency can vary if the supplier doesn't stick to a robust quality management system.
Practical Steps for Validation
Relying solely on supplier claims can cut corners but opens the door to bigger setbacks. Good research groups insist on running their own purity checks—especially for critical measurements or high-value applications. Running a fresh HPLC or NMR is hardly extra work compared to fixing data ruined by contamination. Storing in airtight, light-blocking containers and working under dry conditions keeps products closer to their original grade for longer. Simple but consistent habits, like clearly labelling opening dates and keeping minimal quantities on hand, stretch both budget and reliability further.
Lessons From Real-World Experience
Those who treat purity as an afterthought often fall behind. Collaborations break down, grant reviews falter, and commercial partners grow cautious. Over time, investing in high-grade, well-documented sources pays back in reproducibility and confidence. There’s no replacement for judging suppliers by how they document provenance, batch records, and chemical analysis—trust here gets built over many transactions, not a single smooth delivery.
Bridging Knowledge and Safety
Science labs and manufacturing plants use a pile of chemicals every day. Some fly under the radar, but a chemical like N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide stands out for its unique uses and the care it demands. Over the years, a few simple practices have made life a lot easier — and safer — whenever people work with specialty salts like this.
Making the Storage Work
Every workplace that handles chemicals fights the battle of where and how to store them. Many assume that tossing a bottle onto a shelf works for everything. A mistake like that tends to bite back. In my own experience, skipping steps for “just a minute” because you’re in a rush can quickly turn into hours of cleanup or worse.
So what works? Start with a cool, dry spot — always out of direct sunlight. Many organic salts, including N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide, break down if exposed to moisture or strong heat. Humid spaces can trigger clumping or unwanted reactions. Ventilated cabinets help keep fumes from building up. A good practice is to use containers that close tightly, reducing exposure to air.
Handling Means Suited Protection, Not Shortcuts
Gloves become your best ally in handling almost any specialty salt. Disposable nitrile gloves block unintended skin contact. In college, I watched a friend treat PPE as optional and ended up scrubbing a chemical rash for days. Eye protection matters too — safety goggles shield better than basic glasses. Chemicals splash without warning. If you get product on your hands, wash right away with water and gentle soap. Some people use CaviWipes or similar lab-safe cleaners if the chemical touches a work surface.
Strong ventilation doesn’t get enough credit. Anyone working with chemicals in a closed room will recognize that sharp, itchy feeling in their nose or throat after a few hours. Fume hoods or exhaust fans help clear airborne particles before you even notice them. It’s a small fix with huge impact — I’ve never heard anyone regret installing a better fan at their station.
Staying Ready for the Unexpected
No one enjoys fire drills or emergency plans. But over time, drills save real skin. Everyone ought to know where the eye wash and safety shower sit, and not just “in that corner over there.” For most specialty chemicals, a basic spill kit — absorbent pads, gloves, a waste bag — proves worth its weight. The trick is keeping the kit close, not shoved away under a stack of old boxes.
Label everything, every time. Handwritten tags fall off, so print labels and re-stick as needed. Date bottles when they get opened. Older chemicals sometimes degrade, sometimes form odd crystals or gases. Never ignore a container that looks “off”. Tossing outdated stock beats dealing with bigger messes.
Better Habits, Safer Days
People get pulled into shortcuts, but small, regular habits pay off. Know the risks, plan how to handle spills, and protect your skin and eyes without delay. Working alongside others, encourage double-checking each other’s gear and workspace. Over time, these habits shape a safer culture for every researcher, technician, or new hand joining the team. Safety isn’t about paranoia — it’s about taking pride in every day without an accident.
Understanding the Real Risks
In chemical labs, safety doesn’t come from ignoring what we don’t see or feel right away. N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide might not have a household ring to its name, but its profile still raises real questions. This salt, used as an ionic liquid or intermediate in synthesis, lands in the hands of professionals in research labs and chemical production setups. Every time a chemist opens a container of this compound, they face specific hazards that demand respect far beyond just a pair of gloves.
A Closer Look at Hazards
No one wants to be the person who found out too late about hidden risks. Based on both personal experience and the sparse, direct facts shared in safety data sheets and chemical registries, several hazards come to mind. Skin absorption stands out as a likely route of exposure. Many pyrrolidinium salts easily cross skin barriers, and their modifications often tweak their toxicity instead of removing it. A similar backbone found in other ionic liquids, such as N-methylpyrrolidinium derivatives, has shown acute toxicity in vivo studies. In a glassware mishap or splash, skin contact with this chemical could lead to redness or more serious irritation; absorption risks ramp up with longer or repeated exposures. No one calls that mild.
Eyes demand even more caution. A drop in the eye could cause burning or inflammation, and because these salts dissolve quickly, they spread fast and prove hard to rinse out. Respiratory issues—coughing, wheezing, shortness of breath—might not start until particulate forms or vapor happen in warm labs or during disposal.
First-Hand Experience: The Importance of Precautions
I remember a colleague, in a different lab, who got careless when pouring a small ionic liquid—similar structure, different R-group. The spill soaked through her glove and left a red patch that took days to resolve. Nothing about that taught us to take shortcuts. Shortcuts get noticed in chemical safety; they also get punished.
Best Practices in Chemical Handling
Gloves, goggles, and a lab coat sound obvious, and they are. But a nitrile or neoprene barrier gives better protection compared to cheap vinyl gloves, which split easily. Splash-resistant goggles protect better than regular safety glasses, since even one stray drop is one too many. Clear fume hood work pulls invisible vapors and keeps air safer, so lungs stay healthy.
Reading the label—and the safety data sheet (SDS)—before opening any new container helps. Information gets buried or skipped in busy labs. A direct look at the SDS usually reminds me of hidden dangers, especially when the chemical isn’t one I’ve worked with before.
What Can Companies and Leaders Do?
Training only gets real when it comes with stories and lessons from people who’ve seen things go wrong. Management should offer regular practice: real-life demos on spill management, reminders to perform skin checks, and refreshers on PPE standards. Chemical storage can’t get sloppy either. Labeling stays clear, containers stay sealed, and that stops confusion and cross-contamination before it starts.
The Takeaway
Every new material brings curiosity, but safety stands as the base for every real discovery. Better gear, smart habits, and respecting what we don’t know help everyone leave the lab in better shape than they arrived. For N-(2-Methoxyethyl)-N-Methylpyrrolidinium Bromide, a little extra caution costs nothing but saves a lot—from simple irritation to deeper harm nobody wants as a career souvenir.