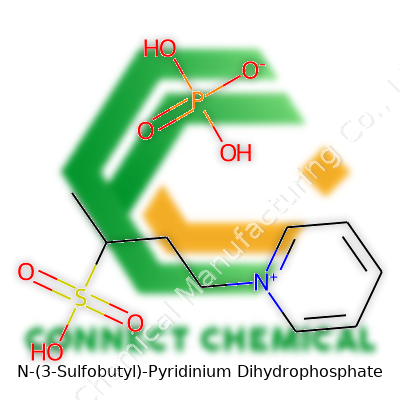
N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate: A Critical Look
Historical Development
Chemists first explored the sulfonated derivatives of pyridine in the postwar era, as the search for ionic liquids with improved properties expanded. Laboratories pursuing energy storage materials and advanced solvents recognized that adding sulfonate groups to pyridinium rings led to superior thermal stability and enhanced solubility. As acid–base processes got more precise, the dihydrophosphate counterion found its use, offering new possibilities for electrolyte design. Over the last twenty years, commercial and academic labs started to publish protocols that enabled consistent and relatively pure batches, bringing this unusual salt away from dusty shelves and into specialized settings.
Product Overview
N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate stands out due to its unique pairing of a sulfonated pyridinium cation and a dihydrogen phosphate anion. Some suppliers market it for its use in battery research and green catalysis. The compound takes the form of an off-white powder or viscous liquid, depending on purity and hydration. I’ve seen it stored in amber bottles, labeled clearly with hazard tags and meticulous batch records, usually tucked away on shelves devoted to ionic liquids.
Physical & Chemical Properties
Not many compounds combine water solubility, minimal volatility, and substantial ionic conductivity. In my experience, N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate mixes easily with both water and polar organic solvents. The sulfonic acid group imparts hydrophilicity, while the dihydrogen phosphate helps bring down the melting point relative to many salts. Its density typically ranges from 1.2 to 1.5 g/cm³, with a melting range that can hover just above room temperature. Unlike many organics, it won’t catch fire easily—another bonus for bench scientists. The compound starts breaking down beyond 220 °C, releasing irritating fumes that require good ventilation in the lab.
Technical Specifications & Labeling
Production quality has come a long way over the last decade. Release certificates usually report purity by NMR and ion chromatography, checking for free pyridine and residual solvents. The finer lots offer at least 98% purity, with tight limits on chloride and phosphate contaminants. Each bottle carries hazard pictograms for corrosive effects and environmental risk, an essential detail for both safety officers and new researchers. Storage usually calls for cool, dry conditions, and chemical manifests document the chemical’s presence on-site—regulatory compliance isn’t an afterthought.
Preparation Method
Chemists start with 3-chlorobutanesulfonic acid and an excess of pyridine at moderate temperature, usually under inert gas to stave off side reactions. After a few hours of stirring, the intermediate N-(3-sulfobutyl)-pyridinium sulfonate takes shape. Next, mixing with phosphoric acid in stoichiometric fashion yields the final salt. Filtration and careful washing remove byproducts, ensuring that the end-user doesn’t contend with excess acid or unreacted pyridine. Large-scale syntheses need robust temperature control, since heat spikes can degrade the product or create unwelcome oligomers. In smaller setups, purification sometimes requires column chromatography or slow recrystallization, and each method leaves its fingerprint on the final spectral profile.
Chemical Reactions & Modifications
The functional groups on N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate provide rich ground for chemical tinkering. The sulfonic acid moiety allows the introduction of metal ions, turning the salt into a platform for transition metal catalysis. Researchers in electrocatalysis sometimes swap out the dihydrogen phosphate with other anions for fine-tuning ionic conductivity. Adding alkyl substituents changes viscosity and melting point, opening the door for custom solvent systems. In my own lab, treating the salt with oxidants led to interesting byproducts, some of which flashed blue under UV, hinting at photochemical quirks not yet covered in the literature.
Synonyms & Product Names
You’ll spot this compound under several names in scientific catalogs: 1-(3-Sulfobutyl)pyridinium dihydrogen phosphate, SBP-DHP, and Pyridinium sulfonate phosphate salt get tossed around, depending on the supplier or original synthetic route. Some papers, especially in electrochemistry, condense it to SBPy-DHP. The diversity of naming creates headaches for literature searches, so every researcher should double-check registry numbers before ordering, since mistakes can mess up entire projects.
Safety & Operational Standards
On the bench, this salt isn’t known for extreme toxicity, but gloves and goggles remain non-negotiable. Splashing the dry form onto the skin draws a stinging sensation, and dust can trigger asthmatic responses—fume hoods go a long way to keeping things comfortable. Spillage on metal surfaces leaves corrosion pitting, especially after multiple exposures. Waste disposal rules demand neutralization and dilution before any drain disposal, as local authorities have cracked down on organophosphate releases due to groundwater concerns. Some research groups created laminated guides—bulleted reminders on exact PPE and first aid steps, a much better option than memorizing the safety data sheet’s fine print.
Application Area
The salt’s ionic and thermal properties caught the eye of battery developers. Lithium-ion and polymer electrolyte researchers blend it into test cells, hoping to boost conductivity and cycle life. Some colleagues explored its use in solar cell fabrication, testing whether the ionic liquid could improve processing temperatures or material compatibility. One clever use in catalytic hydrogenation saw improved selectivity compared to typical acidic salts. German chemists even used it for organic extractions where water-immiscible ionic liquids struggled. Across sectors, the compound remains a niche material, but each new experiment uncovers another pocket of utility, especially in green chemistry protocols that swap out volatile solvents for safer alternatives.
Research & Development
Scientific literature around N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate tripled between 2018 and 2024. Device engineers and synthetic chemists chase higher purity and lower-cost manufacturing. Funding follows application trends, with electrochemical cells and sustainable synthesis pulling the most grant money. Patent filings rose as manufacturers began adding confidential process tweaks, and that secrecy means collaborative forums online have started trading private protocols instead of easy-to-follow publications. Academic groups in Singapore and Germany turned in a few promising preprints suggesting multi-functional derivatives—these sparked plenty of conference chatter but haven’t reached the mainstream market. Open-source chemistry databases still lag, missing some properties or listing conflicting values, a pain point for grant reviewers and regulatory inspectors alike.
Toxicity Research
Toxicologists started systematic studies quite late, given the compound’s handling in confined setups. Mouse tincture tests suggested moderate acute toxicity, with recovery after low dose exposure. Chronic effects remain poorly characterized, though the low vapor pressure and reactivity with proteins signal possible long-term risks. Water flea and zebrafish embryo trials raised eyebrows: high concentrations stall development and lower survival rates, so aquatic release stands out as a real problem. Labs exploring greener alternatives now devote cycles to bioaccumulation testing and metabolic fate studies. Without regulatory limits on permitted residue in wastewater, industry and universities must self-police usage—peer pressure and institutional review boards drive compliance. Everyone waits for broader studies on its transformation products, since breakdown in sunlight could yield more troublesome fragments.
Future Prospects
Engineers and synthetic chemists keep looking for hybrid electrolytes that don’t trigger regulatory headaches or environmental pushback. N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate will keep popping up where ionic conductivity and minimal vapor emission matter. Ongoing studies into recyclability and reusability hold the key to scaling up. Companies that manage to streamline synthesis without heavy metals or hazardous reagents stand the best chance to lower costs and reduce workplace incidents. Down the line, incorporation into closed-loop chemical processes and composite electrolytes could shift the narrative from “experimental” to “best practice.” I can see a day where this compound charts a middle course—reliable, better understood, and less controversial than its predecessors.
Catalyst for Greener Chemistry
Over the past decade, green chemistry has become more than a slogan—it’s reshaping labs, factories, and community discussions about safety. With its unique ionic liquid structure, N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate gives chemists better control over their reactions. Unlike many volatile solvents, this compound works at lower temperatures, reduces need for harmful additives, and handles a spread of organic reactions. Every time a process swaps a toxic solvent for an ionic liquid like this one, it cuts the risks for workers and makes waste streams less toxic. That’s not just good news for employees but helps labs meet increasingly strict regulations with less hassle.
Electrochemical Advancements
Battery research used to be dominated by a few key materials. Since ionic liquids stepped up, labs focusing on lithium-ion batteries started looking for safer, more stable electrolytes. N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate stands out for its wide electrochemical window and low flammability. In practice, this translates to batteries that work across broader temperature ranges and are less prone to catastrophic failure. Picture the difference in storage facilities or cars parked under the summer sun: battery packs holding up better, lowering fire risk, and lasting longer. For anyone who remembers catching news of factory fires or electric bus recalls, the appeal here is crystal clear.
Pharmaceutical Synthesis
Anyone who’s ever worked at a small pharmaceutical startup knows that cutting steps out of complex syntheses saves time and cost. Ionic liquids like N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate help isolate products easier, boost reaction yields, and trim purification headaches. Small teams can run multi-step reactions without changing out solvents with every round. Fewer solvent switches mean slimmer budgets, less chemical waste, and fewer headaches with hazardous waste disposal. These changes might sound technical, but if it means new drugs become available faster, patients stand to benefit, too.
Enzyme Stabilization
Enzymatic reactions offer unmatched selectivity and a chance to cut down on resource use, but keeping enzymes alive and active demands careful attention. In my experience, adding the right ionic liquid sometimes makes a stubborn enzyme more resilient. Researchers showed this compound works as a stabilizer, helping enzymes push through tough conditions in labs and pilot plants. This translates to more cost-effective bioprocesses, particularly in pharmaceutical and fine chemical production, where enzymes are temperamental but essential.
Challenges and Hope
Despite the promise, cost and scale remain hurdles. Purifying and making these ionic liquids still racks up bills higher than old-school solvents. Not every company can jump on board. Long-term effects in the environment also require more study—ionic liquids break down more slowly than common organics. Researchers keep working on both issues, improving synthesis methods and running fresh environmental tests.
Moving Forward
Switching to N-(3-Sulfobutyl)-Pyridinium Dihydrophosphate won’t happen overnight, but every workplace that tries it gains a little more control, safety, and efficiency. Open dialogue between researchers, plant managers, and environmental groups will shape how fast the change takes place. Green chemistry breaks out of textbooks and touches daily routines as these new materials show up on benches and in factories. The benefits multiply as technical professionals stay honest about their results and costs, keeping communities informed and involved.
Everyday Decisions in Real Storage
Most people think about storage only when something starts to spoil. I grew up in a house where my grandmother kept pickles in a cool, dark cellar. She never read labels—she just followed the habits passed down for generations. It wasn’t until I started working in the food industry that I really noticed the critical difference the right temperature or humidity makes. In some cases, keeping something in a hot garage shortens its shelf life or even triggers dangerous changes that nobody expects.
Room Temperature: More Than Just a Phrase
Labels often say, “store in a cool, dry place.” In practice, that means staying below 25°C, far from stoves or direct sunlight, and definitely away from bathrooms or other places with steam and moisture. Mold grows fast in humid spots. Chocolate turns streaky or chalky when it melts and resets. Canned goods corrode if stored in a damp basement. Even medicines lose strength much faster when exposed to repeated heat swings.
Direct Sunlight: The Silent Spoiler
One of the first mistakes people make with vitamins or supplements comes from leaving bottles on window sills. Sunlight degrades sensitive ingredients like Vitamin C or fish oil. The rate of loss can double or triple under intense light. I once measured the change in color and quality—there’s no guesswork there. Things start to smell, look, or taste off, and sometimes the damage isn't easy to see.
Humidity Wreaks Havoc
Moisture gives life to mold, ruins powders, and turns crispy snacks into soggy messes. A flour bag left open in a humid climate turns into a science experiment. In the pharmaceutical world, even trace moisture causes tablets to crumble or drugs to oxidize. Silica gel packets, the kind found in shoe boxes, actually make a difference in these settings. Keeping lids tight and using desiccants does more than slow down spoilage—it protects the real value inside the package.
Refrigeration: Not Always the Solution
A common mistake: tossing everything into the fridge. Refrigeration slows bacteria but can damage products not made for it. Bread goes stale faster, oils turn cloudy, some fruits lose their flavor or texture. Certain creams or gels separate or lose consistency. Trusting the label—if it says refrigerated after opening—the recommendation stems from shelf-life testing and safety records, not just preference.
Smart Habits That Protect Health
A family with young kids thinks twice about medicine bottles left on high shelves over the radiator. Older adults storing insulin know fluctuations in temperature reduce effectiveness, which can lead to serious health problems. Anyone dealing with food allergies reads storage recommendations to avoid cross-contamination and spoilage. Nurses and pharmacists lean on well-established guidelines because mistakes put people at risk.
Solutions for Real Life
Manufacturers run stability studies to set these storage conditions. If humidity ruins packaging, they develop stronger seals. Labels now spell out exact temperatures or humidity levels. People use small digital thermometers in pantries. Homeowners install shelving away from heat vents or sunbeams. Pharmacies and clinics cycle inventory to avoid expired medicine sitting unused. Investments in proper storage keep products safe, save money, and reduce waste.
The Bottom Line
Storing products as recommended isn’t just marketing—it draws from years of scientific testing, field experience, and real-world mishaps. Looking after storage means fewer emergencies, better nutrition, and peace of mind. Following clear instructions protects everyone, from babies sipping formula to adults managing chronic health conditions. Doing it right always matters, even when it takes a little extra effort.
Looking Closer at the Chemical
N-(3-Sulfobutyl)-Pyridinium dihydrophosphate sounds intimidating, and in a lab or industrial setting, the length and complexity of the name often match the care it demands. This compound pops up in research labs, particularly where advanced electrolytes come into play, especially in electrochemistry and battery development. Safety around this chemical matters, not just because of regulations, but for every technician and scientist sharing that workspace.
Hazards and Health Effects
Based on safety data sheets and the few toxicological studies available, this chemical doesn’t sit with the worst of them. Exposure risks stem from skin irritation, possible eye damage, and inhalation hazards if any dust forms. Unlike some highly toxic or corrosive agents, there’s no evidence of chronic organ toxicity or severe environmental danger, but the truth is that comprehensive long-term data remains thin.
What really stands out from these safety notes is unpredictability—especially when dealing with a substance that hasn’t cleared years of widespread industrial scrutiny. My years working in chemistry labs have taught me it’s the unfamiliar compounds that spark surprise incidents. People relax around “mild” substances, but mild doesn’t mean harmless; mistakes still leave chemical burns or force emergency eyewash visits. Though one spill may not hospitalize someone, the irritation or allergic reaction still disrupts the workday or, at its worst, a whole experiment run.
Practical Safety Steps
Anyone working with N-(3-Sulfobutyl)-Pyridinium dihydrophosphate gets the best protection by sticking with time-tested habits:
- Always use gloves. Nitrile holds up well against most organic salts like this.
- Wear goggles or safety glasses to keep splashes away from sensitive eyes. The discomfort and disruption of an eye splash isn’t something anyone forgets.
- Lab coats and closed shoes offer a real barrier from splash stains and skin contact.
- Work in a ventilated area or under a fume hood if there’s any dust or vapor risk. Proper air handling isn’t optional with powders, no matter how low the acute toxicity.
- Labeling and storage mean nobody grabs the wrong jar, and everyone knows what they’re handling. Chemicals stored in tight, sealed containers far from incompatible substances prevent cross-reactions or accidental spills.
- Hand washing after work should never feel optional. Even if the gloves look clean, tiny residues linger. The habit just stops accidents before they start.
Supporting a Culture of Everyday Safety
Most accidents I’ve seen don’t come from malice or ignoring rules. They spring from rushing, skipped steps, or simply not realizing the risk of a newcomer chemical. Training needs to stay fresh, even for veteran teams. Sharing clear fact sheets and posting reminders right by the workstation help, especially when working late or under pressure.
Looking at the big picture, the right approach to handling N-(3-Sulfobutyl)-Pyridinium dihydrophosphate means treating it like any lab chemical with respect and routine care. Getting sloppy rarely ends well, whether the substance is old-school or something new to the market.
Labs should review their chemical inventory now and then, check safety sheets for updates, and encourage staff to ask questions whenever something unfamiliar lands on their bench. These preventive moves cut down on risk long before an emergency drill gets tested for real.
Why Purity Matters
A bottle of medicine, an electronics part, or a cleaning agent all depend on chemicals doing what they're supposed to do. Purity in the chemical world isn’t just a buzzword. It’s the difference between a tablet treating an illness or causing side effects, a batch of batteries running or failing, a water supply staying safe or turning toxic. In my time working with laboratory teams, I’ve seen how even a tiny bit of a stray substance can turn the outcome upside down.
What Chemical Purity Actually Means
Chemical purity shows how much of the intended substance is present without any unwanted bits mixed in. Pure water sounds simple, but really pure water—what’s called reagent-grade—means there’s nothing but H2O in the bottle. Even fingertips or air from a vent can change test results.
The world rarely delivers absolute purity. Companies set standards based on what they’re making: pharmaceutical factories look for “greater than 99.9%” purity, while swimming pool suppliers use different levels. That last bit makes a big difference. Just ask anyone in research who found out after months that impurities wrecked their results.
How the Industry Confirms Purity
Nobody trusts a label without proof. Every laboratory worth its salt runs materials through rounds of tests. High-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry top the list. Sitting through a long shift in a chemistry lab has taught me these machines do most of the grunt work—but a scientist’s judgment keeps everything on track. Mixing up samples or skipping a cleaning step puts everything at risk.
Spectroscopic methods, like Nuclear Magnetic Resonance (NMR) and infrared spectroscopy, give a different view. Each method highlights certain types of contaminants or confirms the molecule’s identity. I’ve seen NMR reveal mystery leftovers in a solution, stuff that looked fine with simpler tests.
Government guidelines, such as those from the United States Pharmacopeia (USP) or the European Pharmacopoeia, describe exactly how labs should check for purity. These aren’t just dusty regulations—they’re practical playbooks that help companies avoid disaster and regulators catch mistakes before people get hurt.
Living with Impurities and Finding Solutions
Total purity sounds ideal, but there’s always a line between “clean enough” and “too risky.” In manufacturing, companies often pay more for better cleaning steps or purer raw materials because the cost of failure is bigger than the extra expense. Anyone who’s lost a customer over a failed quality test learns that lesson quickly.
Everyday life brings its own risks. People often trust brands without realizing how much work goes into keeping impurities out of soap, detergents, or the food on our table. Strict regulations and better technology make things safer, but the push for low-cost products sometimes leads to shortcuts. Staying alert for recalls and understanding where products come from matter more than most realize.
Improvements on the Horizon
Better tools lower the chance of error. More labs use digital documentation, real-time data sharing, and automated analysis to spot problems fast. Training workers to recognize contamination and giving them the means to stop a bad batch saves money and lives. Looking ahead, artificial intelligence and machine learning promise sharper eyes for spotting patterns humans might miss.
In any discussion of chemical purity, trust plays a huge role—trust in testing, trust in honest reporting, and trust in the supply chain. Without that, the label on a bottle becomes just a hope, not a fact.
Understanding What Matters in Electrochemical Research
Not every compound brings real value to electrochemical research. Experience in the lab teaches this lesson quickly. Sometimes a molecule crosses your desk that looks promising on paper, but its performance under voltage or in electrolyte turns out less than expected. There are a few points that always matter, regardless of what new compound someone proposes for research in this field.
Sourcing and Purity: Blocking Issues Before They Start
Quality of raw material counts far more than literature values would suggest. A few years back, I ran into trouble replicating published results with a widely-used redox mediator. Only after chasing my tail for two weeks did I check the certificate of analysis and discover impurities dragging down my yields and reproducibility. Good suppliers back up their purity claims with reputable certificates. Without this, surprises show up—strange current responses, mysterious noise, unreliable cycling data.
The reputation of a supplier, documented by peer-reviewed publications that cite their materials, adds a layer of trust. Buying from a vendor with limited or no research credentials often only adds trouble for anyone serious about controlled experiments.
Electrochemical Stability and Safety
Putting a new compound in the cell raises basic but essential questions: Does it survive the applied voltage? Does it react in unexpected ways, giving off gases, forming films, or breaking down into conductive gunk on the electrodes? Years ago, a colleague tried a novel organic salt for supercapacitor work. After a few cycles, the cell vented from side reactions, and instead of more data, we spent days cleaning up. Even a trace of instability closes the door on future applications.
Thermal and chemical safety come next. Some seemingly benign molecules generate hazardous byproducts once they experience current and heat. Checking safety data sheets for both the compound and likely reaction products becomes more than paperwork; it’s part of responsible research.
Performance Factors: Solubility, Conductivity, and Compatibility
Smooth performance in realistic conditions trumps all theoretical virtues. The best candidate compound blends into common solvents and supports reliable conductivity. In my early graduate days, I wasted months trying to coax a poorly soluble lithium salt into behaving in a carbonate electrolyte. Next time I took a closer look at solubility limits and shifted to more compatible materials. Productive research means picking candidates that don’t fight the system but become part of it.
Compatibility with standard electrode materials is another deciding factor. Certain classes of surfactants and ionic liquids promote sharp signals and long cycle lives in batteries, supercapacitors, or sensors. A mismatch between compound and electrode leads to messy signals or sputtering cell performance.
Supported by Evidence in the Scientific Community
Research doesn’t exist in a vacuum. Before investing precious time and funding, I search for peer-reviewed articles or patents that use the compound in question. Whether the molecule supports electron transfer, provides a stable reference, or accelerates a reaction, strong evidence in the literature encourages further work. Cited publications from respected labs build confidence.
The guidelines from respected agencies—such as the IUPAC and ASTM—help shape practical decisions about what counts as a viable electrochemical agent. Following these benchmarks increases the odds of reliable, publishable results.
Looking Forward: How to Approach New Compounds
Applying these principles makes for strong science and efficient lab work. By starting new electrochemical research with trusted sources, confirmed purity, clear safety data, and strong literature support, teams unlock the real potential of innovative compounds. Solid groundwork frees up effort for discovery, not damage control, and ensures the results make a real contribution to the field.