A Look at N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate: Science, Safety, and Real-World Perspectives
Historical Development
N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate represents a curious stop along the path of ionic liquid discovery. Chemists eager to understand solubility and create alternative reaction media started tinkering with similar molecules back in the 1980s. At that time, people didn’t use the phrase “green chemistry” much—researchers just wanted tools that handled tricky chemical transformations or allowed for easier purification steps. The push for ionic liquids with better water solubility led to creative work with sulfonated side chains, and phenylpyridinium structures piqued particular interest thanks to their stability and affinity for polar and non-polar partners alike. The addition of a trifluoroacetate anion didn’t stem from trend or hype. It solved a practical issue: balancing the cation's hydrophilicity with an anion that boosted both solubility and thermal stability. That hybridization—scientific, pragmatic, and demand-driven—echoes the best of chemical progress.
Product Overview
People working in analytical chemistry or advanced material synthesis come across this compound under a handful of trade names, but the story never changes. N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate acts as an ionic liquid salt, favoring broad liquid ranges and low melting points. Its ionic character makes it friendlier to biological macromolecules than many classic salts or organic solvents. Technicians in mass spectrometry labs appreciate the role it has carved out as a matrix additive that often helps with better proton transfer and smoother spectra, especially in delicate samples. The salt appears as a colorless to faintly yellow viscous liquid, sometimes showing trace color due to small impurities or aging under light. Hydraulic engineers, bioanalytical scientists, and organic chemists still find new tricks and turns for this molecule, and manufacturers keep up with jurisdictional safety and labeling demands.
Physical & Chemical Properties
You don’t need to be a physical chemist to appreciate what N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate brings to the table. It is dense but flows steadily, with viscosity similar to light syrup. The water solubility approaches complete miscibility, which practically means you’ll rarely wrestle with residual undissolved particles. A measured melting point sits well below 100°C, and its boiling point stretches high enough that thermal decomposition—not evaporation—defines its upper use limit. The ionic strength and low volatility combine to minimize unwanted background noise in sensitive tests. Chemically, it resists most moderate acids and bases, so it won’t break down in the standard workups or buffer environments. Its pyridinium ring lends aromatic character, but the sulfonate tail and trifluoroacetate balance that with a polar bite. Slight smells may come from microcontamination but don’t serve as a reliable indicator of purity.
Technical Specifications & Labeling
Chemistry suppliers rarely gamble with inconsistency, especially for research-grade materials. Labels for N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate routinely provide molecular weight (often around 335 g/mol), precise content (typically 98% or higher), and batch information. Trace metals and water content gain particular scrutiny since even a shadow of contamination can up-end a high-sensitivity experiment or scale-up. Bottle labels list storage rules, with directions to keep the salt sealed and sheltered from direct sunlight or heat spikes. Material safety data sheets don’t just pay lip service to hazard codes: they offer first aid, spill management, and PPE guidance that should matter to anyone who values their lungs, skin, or lab bench.
Preparation Method
This salt doesn’t simply fall together with a splash of solvents and a good shake. Synthesis follows a stepwise strategy. Pyridine serves as a starting point, brought to react with 1,4-butane sultone under controlled temperature and with gentle stirring, giving the intermediate N-(3-sulfobutyl)pyridinium inner salt. This gets converted to its trifluoroacetate form by direct addition of trifluoroacetic acid. Purification steps rely on aqueous extractions and careful drying, and vacuum distillation sometimes helps remove trace solvent. Yields hover around 70-85% for careful synthetic runs—not perfect but good enough for those who value reproducibility and reasonable cost. Process improvements often target less solvent waste or skip steps that generate significant byproducts. The lab-grade version commonly sees further filtration through activated charcoal or fine frit glass, giving that signature clarity and readiness for high-performance applications.
Chemical Reactions & Modifications
Creative chemists treat this salt as more than an endpoint—it acts as a building block. The pyridinium ring endures nucleophilic substitution under mild conditions, allowing modifications that tailor electronic or steric properties. The sulfonate group, due to its stability, opens up conjugation potential for materials or surface chemistry. Swapping trifluoroacetate for other counterions paves new avenues, though the original brings the best combo of solubility and compatibility, based on my conversations with colleagues in analytical labs. Its ionic strength and unique solvation help with protein denaturation and enzyme digestion steps. Some ambitious teams experiment with covalent attachment onto silica particles or synthetic polymers, opening up frontiers in sensor technology and rapid diagnostics.
Synonyms & Product Names
No shortage of names follows this molecule around: N-(3-Sulfobutyl)pyridinium trifluoroacetate, Pyridinium, N-(3-sulfobutyl)-, trifluoroacetate salt, and the code-friendly SBP-TFA. Each name echoes either the structural feature set or parent functional groups. Commercial catalogs occasionally assign stock codes or branded tags—none shift the fundamental identity or performance of the chemical inside the bottle. Researchers often shorten the name for lab notebooks, cutting syllables to “SBP salt” or “Trifluoroacetate-modified pyridinium.”
Safety & Operational Standards
This molecule does not show acute hazards in typical use, but anyone who handles chemical salts with poorly understood toxicology knows the routine: gloves, lab coat, and well-ventilated workspace. Acute oral exposure studies report relatively low toxicity, yet respiratory exposure or long-term skin contact hasn’t been exhaustively mapped. Disposal guidance follows recommendations for water-soluble organics, discouraging drain disposal in favor of chemical waste programs, especially for larger quantities or concentrated stocks. The trifluoroacetate component responds poorly to high heat and strong oxidizers, nudging users to store away from open flames or peroxides. Eyes, nose, and open skin deserve protection, regardless of the generally mild risk profile assigned by suppliers so far.
Application Area
Anywhere high-resolution mass spectrometry goes, you’ll bump into this salt. As a matrix additive for MALDI-TOF or electrospray, it helps smooth ionization and tune signal-over-noise ratios for complex biomolecules, peptides, and small-mass polymers. Researchers interested in breaking apart protein complexes or quenching rapid biochemical reactions have reliable results when using this salt, both for its physical properties and ease of rinsing away post-run. Researchers in material science dabble with it, seeking advanced electrolytes or solvent systems where classic ionic liquids may lack water compatibility or safe handling profiles. It slips into diagnostic kits and protein sequencing workflows quietly, with users often discovering its impact only after troubles with lower-grade alternatives.
Research & Development
The R&D world never stands still, and researchers keep poking at how N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate might seed new advances. Current focus stretches from optimizing its use in protein mass fingerprinting to embedding the salt in new nanomaterials for biosensing. Collaborative science turns to modifications that fine-tune its ionic radius or match better with enzyme-loaded platforms. In diagnostic fields, pre-loaded solid supports and stable matrix mixes help clinical labs shorten prep times and boost repeatability. Green chemistry interests push for even safer analogs with equal performance—laboratories have begun experimenting with derivatives that cut down environmental burden without compromising data quality. These studies take years and lots of failed batches, and open access publications in peer-reviewed journals keep the spirit of discovery alive.
Toxicity Research
Long-term studies on this salt remain limited, but what’s in the literature warrants confidence and caution alike. Tests so far reveal low oral and dermal toxicity in rodents, and cell line studies give few causes for alarm inside best-practice boundaries. Researchers still lack long-term ecological fate data for water bodies, so effluent guidelines recommend capture and responsible disposal. No meaningful buildup has been found in common aquatic organisms, but regulatory science moves at a conservative pace. The trifluoroacetic acid byproduct scored some scrutiny as a persistent environmental contaminant, and the salt’s breakdown path remains a topic of graduate dissertations and policy papers. Everyone handling this compound owes careful attention to new findings—today’s green compound rarely escapes tomorrow’s critical re-evaluation.
Future Prospects
Researchers looking to improve both analytical results and sustainability keep a close watch on this and related pyridinium salts. Ongoing work aims to design salts with even finer control over pH, ionic strength, and protein denaturation properties for next-generation mass spectrometry. Companies pursuing cheaper and lower-toxicity production routes seek catalysts that speed up synthesis and shrink waste. Environmental outcomes matter as much as performance, and early tests of biodegradable or less persistent counterions enter the spotlight each funding cycle. Many labs now push to integrate this class of compounds into point-of-care diagnostics, facilitating rapid, reliable biomarker detection in settings far from major urban hospitals. New surface chemistry applications, like immobilized sensors or thin-film electronics, wait on the right blend of cost, performance, and environmental stewardship. Progress depends on the interplay between careful scientific groundwork and practical feedback from the bench—where the next surprise or solution always feels one experiment away.
One Small Compound, Big Job in Mass Spectrometry
You don’t run into the name N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate at the corner store, but folks in analytical chemistry circles pay it a lot of attention. Working in the lab, I’ve seen this chemical shine brightest during tricky protein and peptide analysis. Here’s why it ended up so valuable: it solves real pain points in mass spectrometry, especially with biomolecules that don’t behave.
The Tale of Dirty Samples and Clean Signals
Taking a protein sample—especially anything pulled from cells or tissue—and tossing it onto a mass spectrometer can get messy. Salts, detergents, and all kinds of contaminants stick to your proteins, trashing your data. Electrospray ionization or MALDI (Matrix-Assisted Laser Desorption/Ionization) tends to amplify those problems, sometimes even giving you false negatives. The biggest headaches usually come from charged molecules lurking where you don’t want them, leading to poor ionization or noisy backgrounds.
Here’s where N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate steps in. Chemists use it as an ion-pairing reagent or a sample prep additive. I saw this compound used regularly when prepping samples for mass spectra, especially with peptides that refused to behave. It binds up with interfering salts and surfaces, keeping those from gunking up the ionization step. As a result, signals get clearer, detection of low-abundance proteins improves, and post-analysis sifting through data feels a bit less like wading through mud.
A Closer Look at How It Helps
Working with proteins, I noticed a jump in quality whenever we tossed this pyridinium salt into our prep line. Its negatively charged sulfonic acid group grabs onto positive ions left over from buffers. At the same time, the trifluoroacetate part helps keep the interaction stable but not so tight that it ties things up completely. Mass spectrometrists often look for such a sweet spot, and that’s where this molecule fits in.
Outside my own work, the literature backs these observations up. Analytical Chemistry published several studies highlighting how N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate kicked up sensitivity for peptides and helped in “top-down” protein sequencing. Less background noise meant more confidence in what you’re actually seeing. Given how much pharmaceutical labs and disease researchers lean on clean protein data these days, getting reliable, repeatable results matters more than ever.
The Real-World Impact
The bottom line: this compound makes tough protein and peptide samples amenable to mass spectrometry. For people trying to study complex protein mixtures—say, from tumor samples or old tissue slides—reliable signal means more confident biomarker discovery, which ends up driving better diagnostics and research. From a practical standpoint, it trims down wasted runs on the instrument, saves time on repeat analyses, and cuts through some of the frustration that comes from struggling with dirty data.
For all the jaw-breaking names in chemistry, a small tweak in prep workflow, like adding N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate, can make the difference between hours lost and hours well-spent. That’s a result any scientist can appreciate: less mess, clearer data, and more focus on the big questions rather than the background noise.
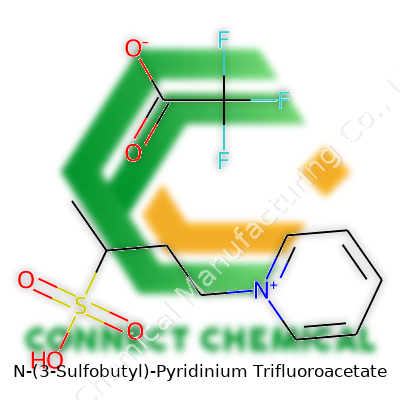
The Backbone: Pyridinium
To get a grip on N-(3-Sulfobutyl)-pyridinium trifluoroacetate, it pays to start with the basics. Anyone familiar with organic chemistry will recognize pyridine, a six-membered ring that borrows a nitrogen for one carbon. Add a positive charge by attaching something to that nitrogen, and you land on pyridinium—a regular in laboratory experiments and pharmaceutical work. Pyridinium brings unique electronic properties to a molecule, able to shuffle electrons in a way that smaller, simpler compounds just can't pull off.
Attaching the Chain: 3-Sulfobutyl
Now, stick a four-carbon butyl chain on that ring, right at the nitrogen. The name tells us it's a 3-sulfobutyl—meaning a sulfonic acid group (-SO3H or as the charged sulfonate, -SO3-) sits on the third carbon. This group drags the molecule deep into the world of water solubility and ionic interactions. In practice, it opens doors for work in aqueous environments and gives the compound a useful profile for separating other molecules or guiding chemical reactions. It's not an everyday decoration for pyridinium, but the presence of sulfonate adds a sort of chemical 'handle' that is almost impossible to ignore—hinting at both reactivity and safety in diverse settings.
The Counterion: Trifluoroacetate
Next, we bump into the trifluoroacetate anion—CF3COO-. Both trifluoromethyl and acetate are stalwarts in chemistry labs, but together they produce a weakly coordinating anion quite comfortable in both polar and non-polar environments. With trifluoroacetate as the counterion, the salt formation boosts stability and usually increases the range of environments where the molecule dissolves. Trifluoroacetate can sometimes influence reactivity in organic reactions or help drive solubilization of certain proteins, especially in tricky separations.
Putting the Pieces Together
You put it all together and end up with a positively charged pyridinium core, a four-carbon chain drifting away, capped with a strong sulfonate group, and a clean, stable trifluoroacetate pairing up for balance. Structurally, it looks like this:
- Pyridinium ring (C5H5N+) at the center, nitrogen carrying a positive charge
- Butyl chain (-CH2CH2CH2CH2-) attached to the nitrogen
- Third carbon of that chain carries the sulfonate group (-SO3-)
- Paired with the trifluoroacetate anion (CF3COO-)
That detailed structure brings practical consequences. Sulfonate groups love water—great for biochemistry. Trifluoroacetate offers compatibility with reverse-phase chromatography, unlocking applications in proteomics and drug research. The molecule walks a fine line between hydrophilic and hydrophobic worlds.
Why the Structure Matters
Chemists care because slight tweaks in structure cause wild swings in function. That butyl chain doesn't just hang around—it stretches the molecule, potentially allowing it to act as a bridge between otherwise incompatible substances. The trifluoroacetate acts more subtly, paving the way for stable salt formation and blending in with solvents during purification steps. A good structure here can boost selectivity, speed up methods, and reduce toxic byproducts.
In laboratory work, clarity on chemical structure leads to fewer mishaps and more predictable reactions. That pays off in everything from basic research to scaling up production. Leveraging knowledge of charged functional groups, solubility profiles, and counterion choices takes this compound out of the realm of theory and into systems that solve complex separation problems or drive greener, safer synthesis.
For scientists focused on better performance and safer workflows, the lessons from N-(3-sulfobutyl)-pyridinium trifluoroacetate offer up a path: keep your molecular architecture in mind, and let the chemistry do the heavy lifting.
Looking at Chemistry from the Ground Up
N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate isn’t a name you hear every day outside the lab. People working with this chemical mostly focus on analytical chemistry research and industrial lab studies. A story from my own graduate days sticks out—I remember shelves stacked with carefully labeled bottles, each stored for a reason: to keep the experiments honest, the researchers safe, and the results clean. Storing chemicals correctly wasn’t just a rule on paper, it became a habit after one spilled bottle taught our team a costly lesson.
Keeping the Chemical in Check
This compound doesn’t explode at room temperature, nor does it evaporate away. Its biggest risks come from storage that ignores temperature swings or moisture. Moisture sneaks into open bottles and turns powders into sticky lumps; temperature spikes break down sensitive molecules. Glass containers with tight lids work much better than plastic, especially for chemicals with fluoride or acid groups. Placing the compound on a dry, cool shelf, away from sunlight and chemical vapors, limits unwanted reactions. It sounds basic, but these steps have saved countless labs from both ruined materials and hazardous cleanups.
In my experience, one of the biggest risks comes from assuming a generic chemical shelf is safe for any new compound. Labels matter, even when you think you’ll remember what’s what. Mark the date received, the date opened, and any hazards—pencils eventually smudge, so go with lab markers on waterproof tape. And keep an up-to-date inventory so you don’t end up with mystery jars at the back of the cupboard, turning dangerous with each passing year.
Side Effects of Ignoring Best Practices
Accidental mixing of incompatible chemicals—like those containing trifluoroacetate with strong bases—can create toxic fumes. I once heard a story from a neighboring lab where a mislabeled trifluoroacetate container ended up next to a bottle of sodium hydroxide. The quick reaction released harmful vapors, a stark reminder that chemical carelessness can turn small mistakes into major emergencies. Education on safety basics, from high school chemistry onward, prevents such slip-ups.
Solutions That Stick
Temperature control is one best tool. Most chem labs use refrigerators for sensitive compounds. N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate keeps well between 2°C and 8°C, far from the fluctuating heat of the laboratory bench. Avoid the freezer; ice buildup on containers can break the glass and ruin the chemical. Desiccant packs inside storage cabinets trap extra moisture, cheap insurance compared to the cost of wasted materials.
Most labs use digital tracking for chemical storage now. These systems don’t lose sticky notes, and sending reminders for audits keeps dangerous compounds from aging out of safety specs. Having a "no food or drink" policy near chemical storage seems basic until a careless lunch break brings soda cans into the lab fridge. The rules keep people safe, not just the chemicals stable.
Training and Ongoing Care
Investing time in regular training, even refresher sessions, makes the difference between a well-run lab and one that stumbles through emergencies. Make spill kits, goggles, and gloves easy to find. Share lessons from real mishaps, not just textbook warnings. Layer these simple solutions, and any lab—small or large—will keep N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate where it belongs: safe, sound, and ready for research instead of rescue calls.
Looking Beyond the Name
Chemical names often sound intimidating to folks outside the lab. N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate, for instance, doesn't exactly roll off the tongue. It’s used in research, especially in mass spectrometry for proteomics and metabolomics, because it helps dissolve stubborn biological samples. But just because something is useful in science doesn’t mean you want it on your sandwich. The big question is—does this compound pose a threat to your health?
Diving Into Safety Data
Safety information on many specialized laboratory chemicals isn’t always as easy to find as you’d expect. For a start, let’s look for a Material Safety Data Sheet (MSDS) for this compound. MSDS documents tell you what happens if you breathe, touch, or swallow a substance, and how to handle accidents. With N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate, MSDS papers typically flag it as an irritant, especially to the eyes and skin. Accidental contact can cause redness or discomfort, and inhaling dust could lead to coughing. So, lab workers wear gloves and goggles— that’s just standard protocol, not a sign of a doomsday chemical.
So far, there’s no clear evidence of cancer risk, DNA damage, or effects on fertility in humans related to this chemical. That’s a relief, but it probably has more to do with the compound being so new and used in such specialized situations. Lack of proof isn’t a clean bill of health; it just means no one’s shown it’s deadly, yet.
Comparing Risks and Real-Life Exposure
Most people never run into N-(3-Sulfobutyl)-Pyridinium Trifluoroacetate outside a laboratory. Compare this to something like bleach, which you can find under most kitchen sinks. Bleach demands respect—get it in your eyes, and you’ll run for the faucet. Still, people handle bleach at home with minimal drama because they know its hazards. The same goes for this chemical if you work in a lab: keep it off your skin, don’t breathe it in, and definitely don’t taste it.
If you spill a gram in a research facility, nobody calls the hazmat squad, but you mop it up wearing gloves. The worst incidents logged usually mention eye irritation or mild skin rashes—bad enough to warrant caution, but not enough to cause panic.
Addressing the Gaps
What about chronic exposure or environmental effects? That’s still a mystery, since limited toxicity studies reach the public, and most data stays in academic or company files. Chemicals tend to surprise us down the line—look at asbestos or lead paint. Given that, it makes sense to be wary of any compound with a long, scientific name and little public track record.
Institutions can help here. Regular reviews of lab protocols, clear signage, and easy access to safety sheets make a difference. Researchers and lab techs can push for open reporting on near-miss incidents, so patterns emerge before real harm strikes. Government regulators could require more transparency about new chemicals before they become popular tools.
The Role of Trust and Training
I’ve worked with enough chemicals over the years to know that most injuries stem from shortcuts or inattention, not the chemical itself. Training trumps fear every time. Teach everyone handling these materials how to respect them, check their protective gear, and keep an eye out for odd reactions. That way, the scary-sounding stuff stays confined to the lab bench—where it belongs, doing its job without causing surprise trips to the nurse.
Finding the Right Balance with Concentration
Mass spectrometry often works a bit like good cooking. Too much seasoning, and the flavor disappears behind the spice. Too little, and the potential of the ingredients goes untapped. In my experience, additives shape the success of an analysis. N-(3-Sulfobutyl)-pyridinium trifluoroacetate (commonly called SPTFA or Pyridinium sulfonate) stands out as a helper for improving ionization, especially in LC-MS or MALDI workflows.
Labs typically use SPTFA between 0.05 and 0.5 mM for direct protein or peptide analysis. Many settle close to 0.1 mM as their tried-and-true starting point. Digging through literature—and facing a few frustrating weeks sorting out signal loss—I learned that pushing the concentration far above 1 mM actually drowns the analyte signals under a snowstorm of background ions and suppresses proper detection.
Real-World Application: Small Details, Big Impact
During routine runs, I usually noticed the sweetest spot for most peptide samples hit right at 0.1 mM. Protein identifications improved, background stayed calm, and the ion suppression didn’t step on my results. Experts agree: too much of this additive brings diminishing returns. People new to mass spec sometimes think bumping up concentration will boost everything. My own early mistakes taught me a sharp lesson—signal starts dropping past 0.2 mM, and the instrument can see a coating of non-specific junk.
SPTFA carries a charge—just right for coaxing hydrophobic analytes onto the radar in electrospray. In your toolbox, this means higher charge states and sharper spectra from sticky proteins or peptides. Stability stays reliable at lower concentrations, too.
Building Confidence With Facts, Not Just Habit
Some push for more additive because vendor protocols suggest higher or lower thresholds, but reviewing leading publications reveals most stick very close to 0.1 mM. For example, the Journal of Proteome Research and a range of Springer or Wiley articles converge on this concentration for standard analytical workflows. High concentrations only crop up for special tricks, like breaking up nasty aggregates or testing unusual post-translational modifications. Most labs pay attention to cost savings, equipment cleanliness, and instrument downtime, so lower concentrations make life easier.
Tackling Challenges: What Works in Practice
Poor results don’t always mean more SPTFA solves the problem. Look instead for sample prep glitches or problems in chromatography. One time my ion suppression worsened even at 0.1 mM—I found out dirty vials left residue that exaggerated background noise. Cleaning up my prep and using only fresh, LC-MS–grade water fixed it. Another chemist I worked with discovered her peptide mapping runs brightened up by dialing SPTFA from 0.2 to 0.1 mM, not the other way around.
Smart Adjustments for Your Own Lab
Settle close to 0.1 mM as your starting point with SPTFA. Tweak up or down in small steps, but don’t expect wild improvements if the rest of your workflow needs help. Track instrument backgrounds, signal-to-noise shifts, and chromatogram baselines. Good records help spot whether a method change or a sample issue deserves more attention. SPTFA stands out as an asset for mass spectrometry, but only if used thoughtfully and not as a cure-all.