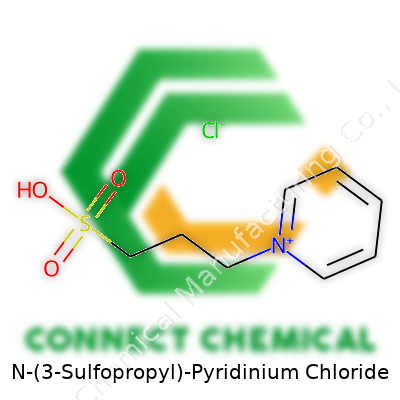
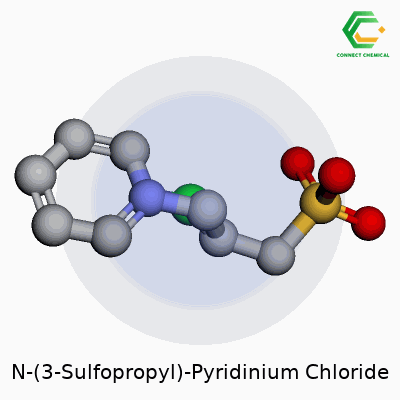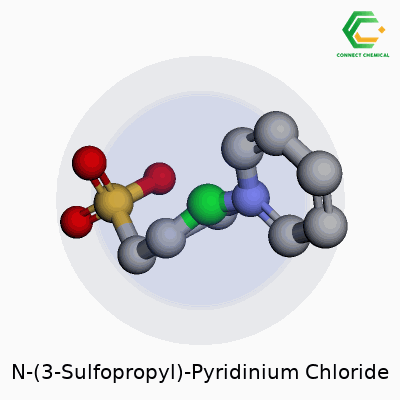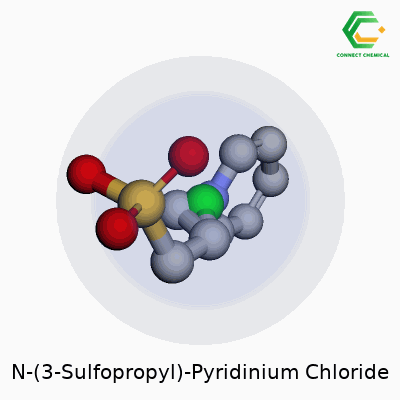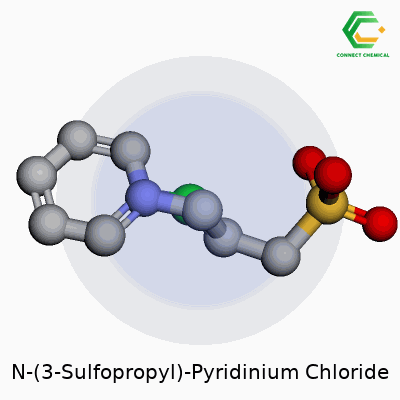
N-(3-Sulfopropyl)-Pyridinium Chloride: Roots, Properties, Utility, and Future
Historical Development
Chemists keep returning to N-(3-Sulfopropyl)-Pyridinium Chloride for a good reason: it’s a compound with a story stretching back decades, woven into the search for better ionic liquids and electrochemical tools. Researchers in the 1960s and 1970s began tailoring pyridinium-based compounds, hoping to stumble upon additives that could smooth out industrial electroplating. Along the way, they found this chemical—compact, water-friendly, and perfect for handling in a lab or a factory. Before the word “green chemistry” echoed through labs, scientists already valued this compound for its salt-like stability and reliability.
Product Overview
This chemical takes the form of a white to off-white powder, sometimes clumping in humid air, with a mild, neutral smell and a bitter taste on the tongue (not that anyone encourages tasting). Companies put it to work in everything from brightening metal surfaces to supporting new-energy batteries. It stands out for its high solubility in water and certain organic solvents. The chloride and sulfonic groups open possibilities that reach from the laboratory bench to large-scale industrial tanks, acting as a link between raw molecular science and products seen on machine parts or in research notebooks.
Physical & Chemical Properties
The formula C8H14ClNO3S packs a punch. Its molecular weight hovers around 239.72 g/mol. At room temperature, it resists melting—a nod to its salt backbone. Throw it in water, and it dissolves quickly, forming a clear, colorless solution. In other solvents such as methanol or ethanol, it’s almost as eager. Because of the sulfonic chain and the chloride counterion, the substance resists hydrolysis and decomposition under normal storage, holding up well in both acidic and basic environments. Engineers like that it doesn’t volatilize easily, so there’s no unpleasant smell wafting around the workplace or lab.
Technical Specifications & Labeling
Manufacturers package this compound under tight moisture controls, slapping labels with its chemical name, batch number, purity (often over 98%), and recommended storage conditions. Containers usually warn against direct contact and spell out its compatibility with nitrile gloves and rubber-lined handling gear. Standard specs require low water content—often under 1%—because extra moisture can trigger clumping or slow down performance in sensitive reactions. Most bottles arrive in light-resistant, double-sealed containers, built to last through transit jolts and long-term storage in warehouses.
Preparation Method
Synthesis usually begins with pyridine, an easily available heterocyclic base. Scientists add 1,3-propanesultone under controlled, cool conditions, stirring for hours so the ring opens up and attaches the sulfonic chain to the pyridinium nitrogen. After that, hydrochloric acid steps in, providing the chloride anion. The mixture gets washed, filtered, and dried, leaving behind a tight, clean product ready for refinement. Yields run high if temperatures stay in check and solvents remain dry, avoiding unwanted byproducts that make purification a headache. Many university and industry labs tinker with the process, scaling up for commercial orders or adjusting starting materials to fit regional supply chains.
Chemical Reactions & Modifications
The molecule’s structure opens up a toolbox of downstream chemistry. The sulfonic acid group can undergo neutralization to form different salts or couple with polymers. Its pyridinium core takes part in oxidation-reduction, quaternization, and even acts as a reactant when researchers build more complex ionic frameworks. Electroplating industries often tweak the compound with minor functional group changes to improve dispersion or tailor its performance toward nickel or copper. Scientists in battery research latch derivatives onto polymer backbones, yielding ionic conductive membranes with improved mechanical strength. With these options, the molecule doesn’t just sit on a shelf; it sets the stage for customization and invention.
Synonyms & Product Names
N-(3-Sulfopropyl)-Pyridinium Chloride goes by several names, reflecting its use across regions and supply chains. Chemists might call it SPP chloride, or sometimes shorten it to just 3-SPPC. Labels may list synonyms like Pyridinium, N-(3-sulfopropyl)-, chloride or even the systematic IUPAC label: 1-(3-Sulfopropyl)pyridinium chloride. On catalog pages, expect to spot product codes sold by Sigma-Aldrich, TCI, or Alfa Aesar, each touting slightly different packaging and purity standards while keeping the molecule at the core.
Safety & Operational Standards
N-(3-Sulfopropyl)-Pyridinium Chloride scores fairly well in terms of safety, but no one should treat it like table salt. Direct skin contact causes mild irritation for some people, so gloves and protective glasses are the right call. Inhalation concerns remain low, mainly due to the low volatility, though dust masks offer further peace of mind during bulk transfers or scale-up experiments. Most manufacturers craft documentation in line with GHS and REACH, pointing out that spills clean up easily with water but leaving leftovers can lead to slippery floors or unwanted reactions with acids or bases. Disposal usually involves controlled landfill or incineration, keeping local environmental guidelines in play. Training workers on chemical hygiene and maintaining up-to-date Safety Data Sheets helps build habits that prevent accidents before they start.
Application Area
Where use matters most, the compound shows up as a supporting electrolyte in electroplating baths, giving a smoother finish to nickel, copper, or gold coatings. In the burgeoning battery field, researchers mix it into electrolytes and polymer membranes for lithium-ion and solid-state designs, chasing improved ionic transport and cycle life. Laboratory chemists rely on it as a mild phase transfer catalyst, while analytical labs sometimes tap its ability to stabilize charged intermediates. Some textile manufacturers try it out in fiber dyeing tanks, benefiting from its solubility and mild chemical nature. Its functions stretch out to polymer modification, corrosion-resistance, and even as an additive in water treatment. Every field wants to harness its adaptability, making new discoveries possible thanks to a molecule that wasn’t intended to be famous but keeps appearing in key recipes.
Research & Development
Cutting-edge work in research circles focuses on enhancing conductivity, boosting environmental compatibility, and lengthening cycle stability in energy storage. Chemists slide N-(3-Sulfopropyl)-Pyridinium Chloride into new solid polymer frameworks, swapping out older, less stable electrolytes. Publications emerging from university and company labs point to breakthroughs in all-organic batteries and next-generation sensors, often made possible by the compound’s ionic nature and chemical flexibility. Incremental tweaks in its side chains or pairing it with other ionic liquids have improved both safety and performance metrics, giving engineers a toolkit for tackling demanding industrial and laboratory applications. As scientists push boundaries in renewable energy and lightweight electronics, interest keeps climbing, attracting grants and commercial funding alike.
Toxicity Research
Researchers have given N-(3-Sulfopropyl)-Pyridinium Chloride a long look under the microscope, verifying its low acute toxicity in mammalian cells and aquatic organisms. Possible risks emerge with chronic exposure—skin reactions or eye sensitivity mostly—steering professionals toward good chemical hygiene. Regulatory groups place the compound in a low-to-moderate hazard category. Long-term animal studies have yet to show carcinogenic potential, but research teams keep investigating for possible bioaccumulation in wastewater scenarios or accidental spills. Consistent review of safety data and honest reporting in scientific journals gives industry leaders, lab managers, and public health officials confidence in handling, disposal, and occupational safety.
Future Prospects
Interest won’t drop any time soon. Battery makers look for better electrolytes that combine stability, performance, and price—N-(3-Sulfopropyl)-Pyridinium Chloride hits those notes. Electroplating shops shift toward less toxic brighteners, and this compound offers a smoother transition without demanding a total overhaul of equipment. Research into next-gen polymers for fuel cells and water purification keeps circling back to its ionic properties. Scientists plan to amplify its potential with green manufacturing and biodegradable derivatives, meeting tighter environmental rules and satisfying end-users who want cleaner, safer chemistry. With so much riding on improved energy storage, affordable electronics, and safer industrial processes, this compound stands ready for fresh rounds of innovation.
One Compound, Many Answers in Science Labs
Most days in a chemistry lab, the difference between a clean result and a blurry one comes down to what goes into the solution. This compound, N-(3-Sulfopropyl)-Pyridinium Chloride, helps scientists get the sharpest answers possible. Typically, it shows up when researchers want better sensitivity and sharper separation in electrophoresis techniques. Anyone who has run a gel knows the frustration of smeared bands and muddy data. That’s where this chemical steps in and makes things clearer.
Why Clarity in Analytical Work Matters
Labs don’t always run like they show in textbooks. Noise creeps into the data, and it’s real easy to miss small changes unless your methods can weed out the clutter. Say you’re examining proteins in a medical sample or checking for contaminants in food. A clean result matters—miss an important signal, and it might mean missing a diagnosis or letting something harmful through. N-(3-Sulfopropyl)-Pyridinium Chloride helps scientists adjust charge and movement of molecules during capillary electrophoresis, sharpening peaks and bringing out details. It acts as a zwitterion, which means it carries both positive and negative charges at once, so it can interact with a wide range of analytes. In practice, scientists find that the compound reduces unwanted interactions at the surface of capillaries, so molecules flow more predictably. That predictability leads to more reliable data.
Direct Benefits in Proteomics and Small Molecule Analysis
Back in grad school, I watched as our research team struggled with faint protein bands. Someone heard about N-(3-Sulfopropyl)-Pyridinium Chloride from a conference. We added it to the buffer system, and overnight, the background cleared up. Spots appeared where before we’d seen only smears. In that moment, I saw firsthand how one reagent can change outcomes. This is not just about personal convenience; the cost of repeating experiments or missing a finding multiplies fast in both time and resources.
Scientists trust data sharpened by this compound, and journals often ask for clear separation in electrophoresis images as part of publication standards. In pharmaceutical work, the stakes run even higher. Analysts need to spot tiny impurities before a drug goes to market, and anything that sharpens peaks on their charts brings more confidence and accountability.
Looking Ahead: Better Tools for Clearer Answers
As research pushes into finding smaller and rarer molecules, the demand for clarity and separation grows. If labs only chase stronger detergents or harsher chemicals, it might damage what they hope to study. Using N-(3-Sulfopropyl)-Pyridinium Chloride means getting better separation without wrecking delicate proteins or peptides. Scientists keep searching for new additives and smarter separation strategies, but right now, this compound fills a real need where sharp and reliable results matter.
Improving scientific analysis often comes down to small tweaks in the workflow. Adopting proven reagents like this one makes the difference between fuzzy and publishable results. In the world of modern science, clarity is more than just a nice-to-have—it’s often the thing that moves research forward.
Understanding Solubility in Everyday Lab Work
Water solubility might seem like chemistry trivia, but it affects so many routines and outcomes that ignoring it usually creates bigger problems. I’ve spilled enough solutions in the lab and watched enough beakers fail to dissolve new powders that the value of water-soluble chemicals speaks for itself. Materials that don’t mix well with water can gum up equipment, produce unreliable results, and slow down research. Finding out if N-(3-Sulfopropyl)-Pyridinium Chloride dissolves smoothly isn’t a side note—it shapes everything you can do with it.
Facts About N-(3-Sulfopropyl)-Pyridinium Chloride
Looking at its structure, N-(3-Sulfopropyl)-Pyridinium Chloride sports a sulfonate group. In everyday chemical terms, that’s a strong clue. Sulfonate groups often give molecules a “hydrophilic” side—the bit that loves water. Pyridinium salts, in general, tend to mix pretty easily with water thanks to that positive charge on the nitrogen atom, and the chloride as a counterion usually doesn’t get in the way.
I checked supplier data sheets from established chemical providers. Most list this compound as “highly soluble in water.” That’s not just a marketing blurb. In practical experiments, a powder dissolves rapidly and forms a clear solution without much coaxing or heating, even at room temperature. Outside the academic setting, I’ve had students work with samples in aqueous mixtures without complaints, which almost always means things went smoothly.
Why Reliable Solubility Matters
Reliable solubility changes workflows. With a water-soluble option, safer protocols follow. Labs avoid using organic solvents that can be flammable or toxic. Accidental spills often turn into cleaning with copious tap water, not fire extinguishers. I’ve seen the relief on a student’s face when a powder dissolves cleanly, instead of caking up somewhere near the spatula.
Solubility also shapes the chemistry itself. Reactions in aqueous settings sometimes run faster or cleaner, and purification becomes less of a headache. Analytical methods, like UV-Vis or HPLC, typically call for solutions, not suspensions or slurries. So, the “soluble” status on N-(3-Sulfopropyl)-Pyridinium Chloride opens the door to a wide range of applications in catalysis, analytical chemistry, and material science.
What Can Go Wrong
Even with “dissolves in water” labels, preparation still matters. Tap water in some regions contains minerals that can react or interfere. Overdosing the powder creates supersaturated solutions leading to precipitation later. I’ve made that mistake: overestimating how much powder fits into a set volume, stirring until my arm hurt, and ending up with a muddy mess. Reliable solubility doesn’t replace careful planning. Stock solutions stored too long can degrade or grow contaminants—something I’ve seen firsthand after leaving a bottle in the back of the refrigerator for a semester.
Better Practices for Handling Water-Soluble Chemicals
Use clean, distilled water for best solubility and long-term stability. Weigh out only what’s needed and keep containers tightly sealed. Prepare fresh solutions before major experiments, and store at recommended temperatures. Teams can build checklists or standard protocols for commonly used reagents to help avoid mix-ups. Training new staff in these basics saves headaches and resources down the line.
Looking at the Bigger Picture
N-(3-Sulfopropyl)-Pyridinium Chloride’s ability to dissolve in water clears the way for plenty of lab work with less risk and more flexibility. Chemical choice can affect everything from daily safety to research outcomes. The details—like reliable water solubility—matter every single day.
The Importance of Getting the Math Right
Understanding the molecular weight of a compound like N-(3-Sulfopropyl)-Pyridinium Chloride goes beyond ticking a box in a lab report. Chemists lean on accurate calculations for everything from weighing reagents to designing safe experiments. A single mistake changes the amount needed, risks contaminating results, or even throws off a whole project. The consequences stretch from wasted resources to compromised safety and questionable science. Getting this number right sits at the foundation of everyday lab work, especially in analytical and synthetic chemistry.
Breaking Down the Structure
N-(3-Sulfopropyl)-Pyridinium Chloride combines a pyridinium ring with a three-carbon sulfonic acid tail and a chloride counterion. The chemical formula, C8H12ClNO3S, sums up its components, but the real lesson comes in remembering not to breeze through the arithmetic. Each element brings its atomic mass: carbon clocks in at about 12.01, hydrogen at 1.01, nitrogen at 14.01, oxygen at 16.00, sulfur at 32.07, and chlorine at 35.45. Adding up these values—eight carbons, twelve hydrogens, one nitrogen, three oxygens, one sulfonic sulfur, and one chloride—delivers a molecular weight of 241.71 g/mol.
Why This Number Matters
Exact molecular weights matter for dosing in synthesis but also in biological experiments. Concentration isn’t just a number—it's the baseline for reproducibility. If a team is testing how a new dye performs in a tissue sample and miscalculates a stock solution, those results won’t align with another group’s attempt to replicate the study. This small error grows when the compound enters market-scale production, where margin stacking can cost thousands of dollars or trigger recalls. Working with substances near their solubility or toxicity limits, a 1-2% miscalculation quickly tips into failure or danger.
Facts Behind Each Digit
In my lab, I’ve watched experienced researchers double and triple-check their math for every new batch. The most seasoned chemists use spreadsheets and double-blind calculations. Errors from relying too much on automated outputs become all too clear when products won’t crystallize or instrumentation starts flagging inconsistencies. Textbooks and databases standardize these values, but trust is built in the details and cross-checks. Relying on software without understanding the underlying calculation leads to clouded judgment. In the days before chemical databases, those extra minutes with a calculator kept entire projects from drifting off course.
Solutions and Good Habits
Sticking to lab discipline saves headaches. Start with a reputable database—Sigma-Aldrich, ChemSpider, or PubChem all report this compound’s molecular weight as 241.71 g/mol. Double-check it with manual addition: walk through each atom’s contribution, write it out, total it up. Use fresh reference material whenever possible. Encourage lab mates to audit each other’s calculations. In teaching undergraduate courses, students often skip these steps, hoping their instruments will catch any error. Only after a few ruined syntheses do those calculation routines set in. Sharing stories of famous laboratory mistakes—where a single decimal point proved costly—turns this invisible math into a shared culture of accuracy.
Conclusion
Working out the molecular weight of N-(3-Sulfopropyl)-Pyridinium Chloride isn’t glamorous, but it’s where most chemistry happens. Behind every successful experiment, those silent calculations form the backbone. Proper habits and solid facts—like the value of 241.71 g/mol for this molecule—make the difference between a trustworthy result and a story you’d rather not share.
Not Just Another Chemical on the Shelf
N-(3-Sulfopropyl)-Pyridinium Chloride keeps popping up in research discussions and laboratory orders thanks to its unique behavior in electrochemical experiments. It’s easy to assume any bottle with a long, scientific label can get the same treatment as sugar in the cupboard. From hard-earned experience in shared lab spaces, though, some materials need much more respect — this compound is one of them.
The Right Environment Makes All the Difference
This substance pulls moisture out of the air. Set it on a shelf out in the open or forget to tighten the cap, and it starts to clump or degrade before anyone notices. Moisture affects its purity, which throws off experiments or makes later quality checks a nightmare. The safest place for this material is an airtight glass or HDPE container. Even a brief exposure to open air in a humid climate can change everything.
Instead of leaving storage location to chance, keep it in a dry cabinet, preferably with a few desiccant packets nearby. My old postdoc mentor once showed me a cracked lid on a similar reagent, and the ruined sample wasted a whole project’s funding for the month. Investing in a dry cabinet might seem like overkill, but the peace of mind and consistent results make it worthwhile.
The Right Temperature: Not Just About Saving Space
Room temperature storage sounds simple, but not all rooms stay stable. Fluctuations in heat, especially near windows or machinery, speed up unwanted reactions. At temperatures above 25°C, the degradation risk rises fast. Best bet has always been a temperature-controlled chemical store or fridge.
Don’t put it near open chemicals or cleaning agents. Cross-contamination is real — one accidental spill, and suddenly a batch meant for precise synthesis ends up full of unknowns. The international guideline from chemical suppliers and lab safety manuals backs this up: isolated storage in a labeled, dedicated container.
Managing Safety and Keeping Compliance
Labs run better when the rules get followed, not skirted. PPE always belongs in the picture when handling this compound — gloves, lab coat, and eye protection at a minimum. Accidents happen, and even a spill during storage or retrieval makes a mess nobody wants. Cleaning up properly and avoiding contact with skin matters for long-term health.
Beyond the physical space, documentation plays a role many ignore. Every time a container is opened or measured out, update the logbook. This way, it’s easy to trace storage conditions and spot issues before they turn serious. Digital chemical inventory tools can help keep tabs on shelf life and flag containers that need replacing.
Reuse, Labeling, and What Experience Teaches
Never reuse containers once they’re empty. Residues linger, and cross-contamination sneaks in. What seems like a minor shortcut can become a multi-day delay if purity is compromised. Use only fresh, labeled vessels — the label trend that includes date received, date opened, and initials of the user has saved me more than once from confusion and argument in group lab work.
All these habits take time to form, but the pay-off is a reliable, high-quality supply that stays usable across dozens of projects. Storage isn’t just about keeping things neat; it’s about taking pride in careful practice and avoiding costly mistakes down the line.
Respect the Substance — Not Just the Label
Lab safety gets skipped far too often, especially when pressure mounts or eyes glaze over after reading dense material safety data sheets. With N-(3-Sulfopropyl)-Pyridinium Chloride, that oversight can create big headaches. This isn’t just a powder you sprinkle in a beaker; it’s a chemical that calls for careful handling, whether working on organic synthesis, electroplating, or as an additive in specialty manufacturing.
Why Proper PPE Always Matters
Years ago, I watched a seasoned technician scoop out a crystalline compound with bare hands, boasting about never needing gloves after hundreds of experiments. Weeks later, untreated dermatitis sent him home for a month. N-(3-Sulfopropyl)-Pyridinium Chloride doesn’t burn skin right away, but repeated exposure can lead to irritation and even long-term health issues. Use nitrile gloves, not latex, since certain chemicals chew through thinner material. Wraparound safety glasses cut the risk of splashes, and a lab coat stops powder from hitching a ride out of the workspace.
Why You Need Ventilation
Many forget that some seemingly stable solids can release dust or vapors. A local chemist down the hall spent hours in an unventilated space last winter, working with a similar quaternary salt. A month later, everyone noticed headaches each afternoon—until an air sample flagged high concentrations of airborne chemical. Fume hoods make a real difference. Regularly check ventilation systems, especially after filter changes or renovations. Don’t store N-(3-Sulfopropyl)-Pyridinium Chloride near acids or heat sources, since unintended reactions can release dangerous fumes.
Dealing with Spills and Disposal
On one rainy afternoon, I found out the hard way how quickly a spill can turn into a problem. This isn’t something you just wipe up with a towel. For N-(3-Sulfopropyl)-Pyridinium Chloride, scoop up the dry stuff with a dustpan, not your hand, and place it in a sealed plastic bag. Any residue needs a thorough wash-down with lots of water. Standard household drains won’t cut it. Dispose of waste through certified hazardous waste services—no down-the-sink shortcuts. Local authorities often publish disposal guidelines. Reach out if you’re unsure; regulations exist to keep workers and downstream water supplies safe.
Keep Your Eyes Open and Communication Clear
Safety training sessions sometimes feel repetitive, but they give teams common ground. A culture where people call out near-misses or update each other on changes in protocol helps. Post updated safety sheets where they’re tough to ignore. Make sure everyone on the team knows when new shipments of N-(3-Sulfopropyl)-Pyridinium Chloride or similar substances arrive, and where they live in the storeroom.
Staying Informed is Staying Safe
Regulatory agencies like OSHA and the European Chemicals Agency track new research on chemical hazards. Subscribe to updates or reach out to local university departments if guidance seems unclear. If symptoms like headaches, coughing, or skin irritation appear, don’t chalk them up to stress or allergies. Have a system in place to report and investigate possible chemical exposure.
Lab work rarely feels dangerous day-to-day, but small safety steps keep projects on track and protect careers. It’s not just about crossing t’s and dotting i’s—it’s about making sure you’re always able to do what you love tomorrow.