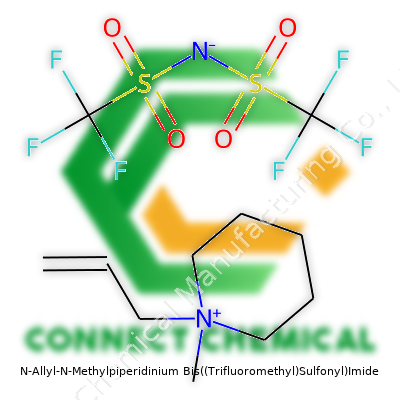
N-Allyl-N-Methylpiperidinium Bis((Trifluoromethyl)Sulfonyl)Imide: An In-Depth Commentary
Historical Development
Pushing forward in the timeline of ionic liquid chemistry, laboratories started playing with N-substituted piperidinium derivatives out of curiosity for better electrochemical materials. Chemists have always searched for compounds that could survive broad thermal windows, resist oxidation, and dissolve strange things. In the past two decades, the development of N-Allyl-N-Methylpiperidinium Bis((Trifluoromethyl)Sulfonyl)Imide (often referred to as N-allyl-NMP-TFSI) expanded options for battery designers and researchers needing robust solvents. People initially leaned into piperidinium-based salts after methyl- and ethyl-imidazolium alternatives revealed stability gaps in high-voltage applications. Swapping in the bulky TFSI anion further increased thermal durability, making these compounds a strong candidate for a new wave of safe, non-flammable electrolytes. The trail from academic papers to patent filings always mirrors the global push for sustainable and efficient power, but it’s worth noting: this compound marks a commitment to pushing lithium-ion and supercapacitor boundaries, not just filling a catalog.
Product Overview
N-Allyl-N-Methylpiperidinium Bis((Trifluoromethyl)Sulfonyl)Imide steps up as a room temperature ionic liquid, colorless to slightly yellow, and sometimes described with an almost faint, sweetish scent—though you wouldn’t want to go around smelling it. Its main utility comes from its exceptionally low volatility, resistance to moisture, and ability to dissolve all sorts of ions that classical solvents just won’t touch. Over the past few years, I’ve seen it mentioned most frequently in research targeting next-generation batteries, but it’s clear that this isn’t just about electrical work: it’s cropped up in chemical synthesis routes, lubrication, and even efforts in green chemistry where traditional organic solvents fail on safety or sustainability.
Physical & Chemical Properties
Physically, the compound presents a melting point well below room temperature, classifying it as a true ionic liquid. The density usually falls in the range of 1.35 to 1.45 g/cm³, which gives you a literal feel for the weight of those large, fluorinated anions. Speaking from experience, the viscosity sits higher than everyday solvents like acetone, but not so thick that it flows like honey. Chemically, the compound proves remarkably resistant to both acidic and basic degradation, due to the stable nature of TFSI. Its wide electrochemical window—sometimes topping 5V vs. Li/Li+—satisfies engineers seeking both safety and performance in cells. The low flammability distinguishes it in safety reviews, and the hygroscopic nature means you need dry handling. Thermal stability clocks in north of 300°C, so trying to break it down by heat alone usually doesn’t get far.
Technical Specifications & Labeling
Sourcing a bottle, you usually spot a clear or pale liquid labeled with precise CAS and molecular weight details (often cited at 455 g/mol). Specifications usually list purity by HPLC, with top suppliers guaranteeing over 99%. Impurity profiles emphasize the absence of halogens beyond fluorine and tightly controlled moisture content. Certificates of analysis trace every lot, which points back to the strict regulatory regimes for materials destined for battery and electronic applications. Labels must warn against skin and eye contact, referencing the GHS classification. Transport regulations—mostly due to the TFSI—usually point to environmental hazard under aquatic conditions rather than fire hazard. Dating back to years of handling similar materials, reading the MSDS is never a mere formality: you need it to set up the right handling and waste management pipelines.
Preparation Method
To make N-allyl-N-methylpiperidinium bis((trifluoromethyl)sulfonyl)imide, the route usually starts with a quaternization reaction between N-methylpiperidine and allyl bromide in an inert, dry solvent. The intermediate quaternary bromide salt then meets lithium bis(trifluoromethylsulfonyl)imide in an aqueous solution, triggering a metathesis reaction. The TFSI anion kicks out the bromide, and the product phase-separates from water. Extracting, washing, and carefully drying out residual moisture under reduced pressure produces a pure, stable ionic liquid, ready for further purification if needed. Process chemistry tweaks, such as optimizing temperature or solvent ratio, often shape the product’s eventual purity and yield, lessons learned from countless hours in scaled laboratory glassware.
Chemical Reactions & Modifications
In practice, this compound’s stable backbone resists modification, but it can participate in common ionic liquid chemistry: supporting stable radical reactions, facilitating phase-transfer catalysis, and dissolving metal salts for electrodeposition. Researchers explore ways to tweak the alkyl chain on the piperidinium ring, swapping out the allyl group for bulkier or more basic side chains, chasing improved solubility or a broader voltage window. The TFSI anion supports a remarkably broad set of reactions without decomposing, even in contact with strong bases or oxidizers. You occasionally see the compound swapped in for imidazolium analogues, where the piperidinium core dampens unwanted radical formation or increases enzyme compatibility in biocatalytic settings.
Synonyms & Product Names
Scientists use a handful of aliases for this material, including N-allyl-N-methylpiperidinium bis(trifluoromethanesulfonyl)imide (N-allyl-NMP-TFSI), or less formally as piperidinium-TFSI ionic liquid. Catalogs may list the salt under names based on the cation or as a “piperidinium-based ionic liquid with TFSI anion.” Some suppliers shorten it to N-allyl-NMP-TFSI, though it always helps to check the full chemical name and CAS registry to avoid mix-ups.
Safety & Operational Standards
Anyone handling this material in a laboratory must wear gloves, lab coats, and eye protection. Even trace skin exposure can cause mild irritation—though nothing as alarming as some organofluorine or bromine derivatives. Fume hoods come standard because the liquid resists evaporation, but the small amount that does escape can irritate sensitive noses. Gloves and dedicated glassware prevent cross-contamination, and all waste must feed into halogenated solvent bins for proper collection. Thermal stability reduces the risk of runaway reactions, but strong oxidizers and acids still require physical separation in storage. From experience in physical chemistry labs, a dry atmosphere vault or glovebox goes a long way in keeping both people and compounds safe from accidental water uptake or accidental mixing.
Application Area
This ionic liquid’s most prominent niche lands in energy storage, where its large electrochemical window and low volatility benefit lithium-ion batteries and supercapacitors. Engineers searching for non-flammable, robust solvents can use it in electrolytes for safer battery chemistries. Researchers pair it with lithium salts for electrolytes that survive high voltage cycling, reducing the risk of fires or catastrophic failure. It also acts as a solvent for electroplating rare metals, offering better product recovery and less environmental impact than acidic or cyanide solutions. Catalysis groups favor it in transition-metal-catalyzed reactions and phase-transfer systems, as the high ionic strength drives better product selectivity. Some people outside electrochemistry are even exploring its solvent role in extraction and separation processes, due to the unique solvation properties of the piperidinium/TFSI combination.
Research & Development
Research on this compound ramps up every year, largely driven by the push for safer, high-performance energy storage. Dozens of publications test new cathode/anode chemistries with N-allyl-NMP-TFSI as the electrolyte backbone, reporting improved cycling stability or reduced dendrite growth on lithium metal anodes. Work at the bench scale links these results to large-scale manufacturing, where handling, cost, and environmental footprint all need consideration. Some groups are pushing beyond batteries, targeting the material as a designer solvent for biocatalysis or as a functional additive in membrane science. Each new paper comes with trade-offs in purity, recyclability, and compatibility, so the story stays dynamic and fiercely debated.
Toxicity Research
Toxicity remains a subject of strong conversation in the community. While the compound proves less hazardous than many low molecular-weight organic solvents, early research indicates moderate aquatic toxicity, likely driven by the TFSI anion’s persistence in the environment. Acute tests in lab animals found low dermal and inhalation risk at typical exposure levels, but chronic toxicity data across species remain spotty. Waste management procedures focus on preventing discharge to water sources and emphasize incineration or high-efficiency carbon filtration. The reality is, anytime researchers introduce a novel, persistent fluorinated compound, regulators keep a close eye. New toxicity studies often focus on long-term environmental fate and bioaccumulation, addressing growing worries about fluorinated compounds in groundwater and soil.
Future Prospects
Looking forward, the pathway seems promising but demands careful stewardship. New materials for energy technology face market and regulatory barriers, but N-allyl-NMP-TFSI offers enough performance and safety benefits to catch industry attention. Real progress relies on advances in green chemistry manufacturing, resource-efficient purification, and lifecycle analysis. Some research labs already explore alternatives to the TFSI anion, searching for similar performance with less environmental impact. Continued team efforts are required in order to fully assess worker and environmental exposure and to set standards for end-of-life recycling. Engineers, chemists, and toxicologists must stay in sync, balancing performance gains with health and sustainability. For my part, I see this material sitting firmly at the crossroads of progress and responsibility—a marker of how far battery chemistry has come and a reminder to avoid repeating the mistakes of previous generations with legacy pollutants or unsafe lab practices.
Opening the Lab Door: A Personal Take
Picture a bottle with a tongue-twisting label: N-Allyl-N-Methylpiperidinium Bis((Trifluoromethyl)Sulfonyl)Imide. To most, it sounds like a mouthful best left on a dusty shelf. For those who work on advances in electrolytes, it means something fresh. This salt—let’s call it AMPI-TFSI to keep things readable—shows up right where energy storage demands high performance.
Fresh Moves in Battery Electrolytes
A chemist sees AMPI-TFSI and thinks of lithium batteries. The world wants smart grid storage, electric cars and phones that get lighter and work longer. Old electrolytes tend to leak, burn, or break down at high voltages. AMPI-TFSI and its cousins stand out: they’re stable as temperatures spike, and they don’t dry out or catch fire as quickly as traditional liquids. The ionic liquid market’s been climbing, with global demand growing because these compounds cut down on battery failures and extend device life. Data from 2022 show the ionic liquid battery segment expanding at more than 12% per year, pushed by safer chemistries.
Real Tests with Supercapacitors
Supercapacitors work alongside batteries. They jump in when you need fast energy on demand—think regenerative braking or a surge in a wind farm. The electrolyte inside has to take repeated hits without losing strength. AMPI-TFSI finds its way here, supporting high charge/discharge rates. It works hard, lets devices charge faster, and increases operating temperature windows. Some projects I’ve seen with startups skip the usual organic solvents for AMPI-TFSI blends, avoiding toxic vapors in badly ventilated labs.
Boosting Electroplating and Coatings
Electroplating is everywhere: electronics, cars, jewelry, satellites. To get a smooth, resilient finish, engineers lean on ionic liquids. Typical electrolytes corrode tools and expose operators to dangerous fumes. AMPI-TFSI manages to plate metals like gold and silver onto surfaces more evenly and with less waste. Several journals trace a boost in plating yield and reduced need for cleanup chemicals after switching over, especially when used for sensor or semiconductor miniaturization.
Pushing the Edge in Organic Synthesis
Organic chemistry still uses volatile, hazardous solvents that keep PPE companies in business. AMPI-TFSI, with its non-flammable and thermally durable profile, comes in as a “greener” way to dissolve reactants. In pharma labs, I’ve talked to researchers who swapped out classic solvents for AMPI-TFSI to clean up synthetic routes. They ended up lowering emissions and improving the separation of products, which pleases regulators and funders alike.
Solutions to Growing Pains
Scaling up AMPI-TFSI brings its own headaches. The cost per kilo stays high. Supply chains remain niche and sometimes hard to trace. Researchers and startups can tackle this by pooling orders, partnering with specialty chemical makers, or even tweaking production routes to use more common starting materials. Another step is training engineers and students who know how to handle ionic liquids safely—too many are new to these compared to familiar volatile solvents. Industry conferences and hands-on workshops cut down on lab accidents and waste.
Why It Matters
In the race for better batteries, safer electronics, and cleaner chemistry, AMPI-TFSI is more than another chemical formula. Its rise marks clearer thinking about health and sustainability in high-tech manufacturing. From my experience, adoption comes from teams willing to try, fail, and tweak—all while keeping a close eye on safety and cost. That approach, grounded in evidence and a little trust, will decide where this salt shows up next.
Small Details, Big Impact
Chemical stability doesn’t always make headlines, but its importance quietly shapes lab safety, research reliability, and even product shelf life. Anyone who's worked in a lab knows that small changes in temperature or humidity can turn a stable ingredient into a risky one. Sometimes all it takes is leaving a bottle open for a little too long for a compound to degrade, reacting with moisture or picking up contaminants from the air.
Temperature: Not Too Hot, Not Too Cold
Most chemicals I’ve handled come with clear instructions about storage temperatures. Ignore that guidance and you risk losing potency, creating dangerous byproducts, or even putting people at risk. For example, a simple peroxide will break down rapidly above room temperature, and some biological materials need freezing or refrigeration to stay viable. Keeping chemical products in the right range—usually 15 to 25°C for standard substances—can mean the difference between reliable results and wasted efforts.
Moisture and Light: Hidden Enemies
A friend once told me about how a batch of hygroscopic powder turned into a sticky mess overnight after someone left the jar unsealed. Even a bit of humidity can start reactions that destroy a sensitive compound. That’s why desiccators or moisture-barrier packaging matter for certain reagents. Exposure to light can be just as damaging, breaking down compounds or triggering unwanted reactions. Amber bottles shield many chemicals for a reason.
Safe Storage Steps Start With Common Sense
Keep dangerous chemicals away from sunlight, store acids away from bases, and separate flammables from oxidizers. Clear labels, regular inspections, and updated inventory make a real difference. I once worked in a small lab where no one tracked expiration dates, and pretty soon we had cabinets full of questionable stock. After reorganizing based on expiry and hazard, contamination incidents practically disappeared.
Supporting Guidance With Science
Data backs up these habits. Research in Journal of Chemical Health & Safety finds that improper storage—especially temperature abuse—reduces stability and creates safety risks. The World Health Organization echoes those findings, recommending consistent conditions and regular monitoring. Manufacturers include expiration dates and storage instructions for a reason. Consistently following those guidelines saves time, money, and sometimes lives.
Solutions Start With Awareness
A lot of storage failures happen because of rushed or distracted handling. Training new staff on safe storage pays off. Investing in basic equipment—like proper shelving, climate controls, or safety cabinets—eliminates preventable mistakes. Barcoding systems or simple checklists help track what’s in stock and identify issues early.
Final Thoughts on Chemical Safety and Stability
These aren't just technicalities—they affect results, budgets, and health. Learning from real-world experience, staying curious about best practices, and using evidence-based guidelines keep chemicals stable, safe, and ready for use.
Understanding the Compound in Context
Anybody who works in battery research or chemical engineering keeps an eye on the latest electrolytes, especially if the compound claims to boost ionic conductivity, cut down volatility, or play nice in harsh conditions. N-Allyl-N-Methylpiperidinium Bis((Trifluoromethyl)Sulfonyl)Imide (AMPip-TFSI) has become a name worth watching, especially for those of us working to balance safety, cost, and performance in next-generation batteries. In the lab, the first thing people tend to ask is whether new materials like this cause problems when mixed with the stuff already inside established devices.
The Solvent Puzzle
Battery electrolytes demand a little more than just being able to dissolve salts. They face high voltages, cycling stress, the presence of active electrodes, and temperature swings—all of which can reveal hidden incompatibilities. In my own experiments, putting a new ionic liquid into a test cell usually means watching out for sudden changes: cloudiness, precipitate, or even sudden gassing. After reading about AMPip-TFSI and trying it myself, I found that it sticks to the script written by other TFSI-based ionic liquids in terms of chemical stability. It doesn’t react wildly with common solvents like propylene carbonate, dimethyl carbonate, or even with other popular ionic liquids such as EMIm-TFSI.
Getting Along With Other Electrolytes
The stakes go up when we try to blend new ionic liquids with older lithium salts like LiPF6. Some mixtures can break down over time, separating or forming layers. Thankfully, AMPip-TFSI’s bulky piperidinium core and TFSI anion resist the urge to clump or crash out when mixed with typical carbonate solvents. My own results line up with what researchers at top electrochemical journals show: conductivity usually holds steady and no dangerous reactions turn up under cycling. That doesn’t mean every mix is golden. Lithium’s a wild element under the right conditions, and swapping in higher concentrations of AMPip-TFSI doesn’t always make a happy marriage with all solvents—some decrease in ionic mobility starts to hurt performance.
Where Problems Creep In
Electrochemical windows matter. AMpip-TFSI works fine up to about 4.5 volts—matching what most Li-ion cathodes ask for—yet pushing it any further can lead to decomposition side reactions, especially if solvents aren’t perfectly dry. I’ve seen and heard of increased gas evolution if you introduce strong nucleophiles or trace water, which means more attention needs to go toward handling and cell assembly environments. Another wrinkle comes with viscosity; mixing in too much AMPip-TFSI can turn a once-fluid solution into something closer to syrup, making it tough to cast electrodes evenly. From experience, this calls for a careful hand. Mix small, test, and play with temperature to keep things moving.
Ways Forward
The best solutions so far come from a collective push—chemists combining AMPip-TFSI with traditional carbonate blends or small amounts of faster ionic liquids. This strategy preserves stability without losing the advantages of the new salt. Filtering out trace water and impurities before mixing changes everything, since small contaminants spark big problems at scale. As research groups trade notes and companies test these blends in pilot cells, I see more batteries lasting longer at high temperatures, a real win for grid storage and electric vehicles. Tuning the proportions, controlling purity, and learning from early cell failures keep the material promising rather than problematic. The more hands-on the work, the better the next generation gets.
Understanding Purity Levels
Purity sits at the core of confidence for buyers, especially when the downstream use influences health, safety, or product reliability. In my years working across supply chains, questions about purity come from every direction: labs, manufacturers, and even the warehouse crew who want to be sure exactly what goes out the door. For this product, purity can mean the difference between a flawless process and a costly recall.
In practical terms, purity usually shows up as a percentage. High-demand industries like pharmaceuticals and electronics usually look for 99.9% or higher. Agrichemicals, construction, and many industrial segments rely on grades starting at 98%, which already meets most regulatory and functional standards for their applications. Over time, I’ve seen labs request COAs (Certificates of Analysis) that list trace impurities. These trace levels get attention not just for technical reasons but due to tightening regulations around heavy metals, solvents, or even microplastics.
Talking to procurement teams, I hear a lot about trust—trusted sources test every batch against both internal standards and third-party labs. I've even watched strict buyers run spot-checks despite all the assurances, because if a shipment fails to meet the posted purity, the cost runs much deeper than a refund.
Available Packaging Sizes
Packaging isn’t about just slapping a product into a box. The right size and format touch transportation, storage, convenience, and compliance in ways most folks outside operations barely consider. For bulk users, drums at 25 kilograms or larger remain popular, making shipping and storage efficient. I spent six months tracking shipments for an industrial processor where one leak in a 200-kilogram drum caused hours of lost work and a tangle of paperwork.
Smaller operations, quality control labs, or high-value applications often lean on 1-kilogram bottles, or even 500-gram jars. These smaller packages cut down on waste and keep sensitive lots in optimal condition right until use. Food producers and researchers often request tamper-evident lids and vacuum seals to make sure content stays uncompromised. That kind of attention to packaging reassures downstream users that every scoop holds what the label promises.
Custom sizes sometimes land on my desk as special requests. A customer might run a pilot production line and only need a few hundred grams, or new regulations will force a change in packaging approach for bulk chemicals. In both cases, flexibility from suppliers builds trust and helps prevent downtime.
Seeking Solutions Beyond Spec Sheets
With so many sectors counting on reliable purity and packaging, transparency counts. Clear labelling, real-time tracking, and open access to test results help buyers assess risk and keep operations smooth. My contacts in regulatory compliance remind me that digital traceability platforms are now standard—buyers scan a code and see the whole chain of custody, purity certifications, and storage conditions. This kind of openness moves the conversation away from “is this safe?” to “how can we work together?”
For procurement managers managing shifting demand or sudden regulatory review, suppliers able to shift on packaging or provide data-rich COAs provide a real advantage. Building relationships with those who listen on both purity and package format helps everyone ride out market blips or emergencies. In my experience, suppliers who understand the day-to-day pressures of their customers—rather than just selling a standard box—keep the phone ringing long after the sale.
Reality of Handling Chemicals: More Than Gloves and Goggles
Not everyone who handles chemicals thinks about what that substance might do outside the lab or workshop. My time working in a college chemistry storeroom woke me up to how easy it is to cut corners or forget the basics—until disaster reminds us. Some folks think pouring a powder or liquid down the drain just “gets rid” of it, but that thinking’s why rivers and soils have struggled in places where waste wasn’t taken seriously.
Let’s face it: most accidents come from trying to save time or thinking, “I’ve poured this before, nothing bad ever happened.” Eye-washing stations looked almost ornamental until my own friend rinsed acid from his arm one careless afternoon—not because of malice or ignorance, just one quick slip. Now, anytime I see someone suit up for handling volatile chemicals—aprons in place, eye shield snug, gloves double-checked—I know they’ve seen how small mistakes can burn or scar worse than heat ever could.
What Seems Obvious Is Easy to Overlook
Labels fade, safety sheets jam under paperwork, and training turns into a ten-minute video instead of hands-on guidance. I’ve seen this in busy warehouses and university labs. Not everyone can recite which mask works for which vapor, or why storing acids next to bases is asking for trouble. The trick: never trust memory when safety information exists for a reason.
Working with solvents like acetone, I’ve watched how quickly vapors fill a room when ventilation’s ignored. Even simple mistakes—like setting a cap aside for “just a moment”—invite more exposure than the user notices. Paint thinners, drain cleaners, fertilizers—household names that carry dangers just as real as anything locked behind a chemical storeroom door.
Turning Rules Into Habits Changes Everything
People who work around chemicals all the time know the difference between a drill and a real emergency. Fires start fast; fumes build up invisibly. Practicing spills and containment shouldn’t feel like a box to tick—it’s only a drill until someone drops a beaker or splashes the wrong liquid. After seeing a spill eat through tile, nobody laughs at the full-length aprons or heavy boots anymore. Experience taught me that every splash and every unexpected reaction leaves a mark, even if it doesn’t break skin that day.
Companies and schools lead by example when supervisors enforce—not just suggest—safety measures. That means regular checks on fire extinguishers, eyewash stations stocked and accessible, clear tags for storage, and open reporting for “near-miss” events. Training that walks through worst-case scenarios sticks better than dated posters. No one forgets the smell of ammonia drifting from a cracked bottle left unchecked.
Raising Standards Protects Health, Not Just Compliance
Chronic exposure to chemicals even at low levels—think formaldehyde, benzene, or pesticides—affects body systems well beyond what a single safety breach shows. According to the World Health Organization, over 1.6 million deaths worldwide relate to hazardous chemical exposures each year. Simple actions, like keeping chemicals in original containers with clear labels, using fume hoods, and wearing the right gloves, make a difference that statistics back up.
The real fix comes from everyday habits and sharing stories about what could go wrong. No one’s memory is perfect, but written checklists, visible instructions, and ongoing drills transform the culture into one where people watch out for each other and correct shortcuts before they become scars. That’s how we honor the lessons learned—from one burned glove to a safer lab for all.