N-Allyl-N-Methylpiperidinium Hexafluorophosphate: An Expert Perspective
Historical Development
In the landscape of synthetic organic chemistry, N-allyl-N-methylpiperidinium hexafluorophosphate emerged out of the search for novel ionic materials with increased chemical stability and unique physicochemical properties. During the latter half of the 20th century, piperidinium-based salts gained attention due to their thermal resilience and broad utility. Early work focused on ammonium and phosphorus-based analogs, but researchers quickly saw the advantage of bulkier, cyclic amine cations. Lab notebooks from the '70s and '80s often feature hand-drawn reaction schemes scrawled alongside warnings about moisture sensitivity. The advent of reliable anion exchange methods in the late 1990s simplified access to hexafluorophosphate salts, making research-grade samples more available. Scientists working with batteries, catalysis, and molecular recognition turned to these salts for improved ionic conductivity and electrochemical windows.
Product Overview
N-allyl-N-methylpiperidinium hexafluorophosphate turns up more frequently as a white or off-white crystalline powder, sometimes in small, sealed bottles wrapped in plastic to keep moisture away. To the naked eye, nobody could tell it apart from dozens of other tetraalkylammonium salts lined up on the shelves of research labs. Behind that bland appearance, you get a combination of organic cation and inorganic anion that slots into processes ranging from non-aqueous solvents to electrochemistry. Chemists value this salt less for what it does on its own and more for the stable platform it offers, letting them tweak everything else in the reaction without worrying about decomposition or pesky side reactions.
Physical & Chemical Properties
With a molecular formula of C9H18N·PF6, the molar mass comes in just above 299 g/mol. This compound dissolves well in polar aprotic solvents like acetonitrile and dimethylformamide. It won’t budge in nonpolar solvents such as hexane. It tends to melt in the 160–190°C range, which speaks to the strength of the ionic lattice. Even holding a test tube of moist air near the sample is enough to warn researchers about slow hydrolysis, as the hexafluorophosphate anion isn’t shy about releasing a faint whiff of HF. Under normal storage, the chemical remains stable for months. The piperidinium core shrugs off mild acids and bases, only succumbing under harsh conditions that break the nitrogen ring.
Technical Specifications & Labeling
From the chemical supplier’s side, each package arrives with batch-specific certificates of analysis, lot number, and synthesis date. A standard label highlights the product name, structural formula, chemical purity (typically above 98% by NMR or HPLC), and moisture content. Infrared and NMR spectra usually accompany the batch paperwork, letting researchers confirm the absence of unreacted starting materials or degraded byproducts. Flammable, irritant, and environmental warnings remind users that neither the cation nor the anion should end up in household drains. I have seen labs pay extra for vacuum-sealed packaging to protect the salt from ambient humidity, especially in regions with high precipitation.
Preparation Method
Lab-scale preparation usually begins with N-methylpiperidine and allyl bromide, generating the quaternary ammonium bromide salt in refluxing acetonitrile. Without elaborate fuss, chemists filter out the crude salt, wash it, and dissolve it in deionized water. Addition of aqueous potassium hexafluorophosphate prompts immediate precipitation of the target salt, which is then filtered, rinsed in cold ethanol, and dried over phosphorus pentoxide. Some procedures feature a further recrystallization step from acetonitrile/ether to ensure maximal purity. This synthetic sequence does not involve any exotic reagents or high pressures, which keeps it accessible for most labs with basic glassware and vacuum line.
Chemical Reactions & Modifications
In practice, N-allyl-N-methylpiperidinium hexafluorophosphate takes on the role of both supporting electrolyte and ionic co-catalyst. The allyl group stands ready for cross-coupling or nucleophilic substitution, letting researchers generate substituted piperidinium derivatives without the need to resynthesize the entire salt. Some teams explore electrochemical reductions, where the molecule’s wide window prevents decomposition at the electrode surface. Others use the cation as a phase transfer agent in biphasic organic reactions. With appropriate reagents, selective de-allylation or alkyl group exchange expands the synthetic possibilities, so this salt often serves as a modular platform.
Synonyms & Product Names
Common trade names include “N-allyl-N-methylpiperidinium PF6” and “AMPipPF6.” Catalogs sometimes lump it under the catch-all “quaternary piperidinium hexafluorophosphates.” Synonyms echo its structure: 1-allyl-1-methylpiperidinium hexafluorophosphate, N-methyl-N-allylpiperidinium hexafluorophosphate, and their abecedarian equivalents. Chemists ordering this material need to double-check CAS numbers (usually 185330-36-1) to avoid confusion with methylallylpyridinium or substituted tetraalkylammonium salts.
Safety & Operational Standards
Working with this salt means gloves, goggles, and a well-ventilated hood. Organic hexafluorophosphate salts can hydrolyze to release toxic hydrogen fluoride under acidic or wet conditions, making acid scrubs and waste collection crucial. I recall fielding several anxious emails from graduate students worried about that faint etching in beaker glass—sure sign of trace HF exposure. The salt itself doesn’t burn easily, but heating or disposal burns unleash corrosive gases, so good lab practice always means chemical-resistant bins, sealed waste bottles, and routine safety audits. Employers push for regular HF safety refreshers, knowing the damage a drop of invisible acid can do to unprotected skin.
Application Area
Applications revolve around the unique cation-anion pairing. In non-aqueous electrochemistry, N-allyl-N-methylpiperidinium hexafluorophosphate boosts conductivity in solvents that lack strong hydrogen bonds. Battery developers use it to stabilize electrolyte formulations for prototype lithium or sodium cells. Drug discovery teams see value in its piperidinium backbone as a building block for nervous system agents. Nanomaterial scientists rely on its ionic strength for templating layered materials and driving self-assembly processes. Catalysis specialists experiment with these salts as phase transfer agents, using their organic character to bridge water and oil phases for stubborn C–C coupling reactions. Lab experience demonstrates that yield and selectivity often improve with careful tuning of this component.
Research & Development
Ongoing research focuses on mapping the subtle influences of different cation and anion pairs on electrochemical properties. Some projects investigate substitution patterns on the piperidine ring, introducing electron-withdrawing or electron-donating groups to optimize solubility, redox stability, and reactivity. Scale-up studies now compare batch and flow synthesis routes to improve efficiency, reduce waste, and minimize environmental impact. Collaborative efforts across universities and startup labs push for tunable ionic liquids with new applications in clean energy and sustainable production technologies. Reviews in major journals routinely highlight the importance of platform molecules like N-allyl-N-methylpiperidinium hexafluorophosphate in developing smart materials and functional interfaces.
Toxicity Research
Actual toxicology studies trace their roots to the growing awareness that both cation and anion may interact with biological systems in unexpected ways. Acute exposure in rodents produces mild, reversible symptoms at low doses but neurological effects and organ stress at higher concentrations. The major risk involves chronic exposure to the hexafluorophosphate anion, which degrades into fluoride under typical environmental conditions. Waste management policies increasingly call for closed-loop recovery or licensed disposal to avoid waterway contamination. Cell culture studies report moderate cytotoxicity, mostly tied to disruption of membrane integrity. Guidelines suggest treating all ionic organic salts with the same respect due to the unpredictable bioactivity that comes with charged, lipophilic molecules.
Future Prospects
The push for safer, greener chemistry will shape future directions for N-allyl-N-methylpiperidinium hexafluorophosphate. Teams engineer new analogs with less toxic counterions or biodegradable cation scaffolds, aiming to preserve function without environmental baggage. Improvements in industrial production, like solvent-free synthesis and energy-efficient purification, promise cost reductions and lower carbon footprints. Energy storage systems look to next-generation ionic liquids to deliver fast-charging, high-capacity batteries, and this salt stands among the contenders. Synthetic chemists anticipate deeper insights from computational models that predict reactivity and biological activity based on subtle structural tweaks. Regulatory shifts, driven by toxicity data and lifecycle assessments, will push companies to weigh performance against safety and sustainability. My own experience shows the power of broad collaboration across disciplines—electrochemists, toxicologists, environmental engineers—to unlock the full potential while minimizing risks associated with this versatile compound.
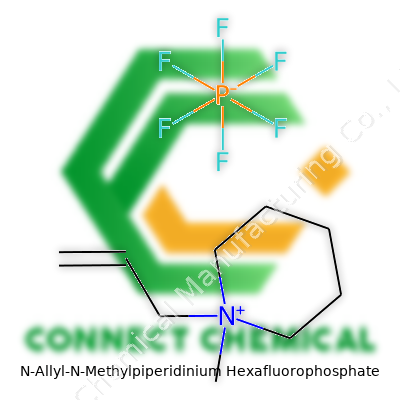
Understanding the Molecular Backbone
N-Allyl-N-Methylpiperidinium Hexafluorophosphate might sound complicated, but breaking it down makes it approachable. Picture the piperidinium core: a six-membered ring with five carbons and one nitrogen, which gives the compound its stability and helps guide its reactivity. Attach a methyl group to that nitrogen — a single carbon and three hydrogens — and now there's more flexibility and different chemical behavior. By adding an allyl group (C3H5), the structure starts to look like something practically useful, not just a scientific mouthful. The molecule stands out in electrochemistry because it manages to balance stability against the strong reactivity often needed in battery research or synthetic chemistry.
The Role of Hexafluorophosphate
Pairing the piperidinium ion with a hexafluorophosphate (PF6-) anion sets the stage for this compound’s real-world role. Six fluorine atoms circling a phosphorus atom don't just look cool on paper — this arrangement resists both water and weak acids. I remember working with hexafluorophosphate salts during a project on ionic liquids. These materials barely blink in the face of humidity, which helps explain why N-Allyl-N-Methylpiperidinium Hexafluorophosphate gets chosen for electrochemical applications where moisture wreaks havoc.
Why It Matters in Everyday Research
Choosing the right chemical for a job isn’t just about what’s on paper, but what happens bench-side. In battery development, materials that keep ions moving smoothly without breaking down extend device life and reliability. Ionic liquids based on piperidinium ions, especially ones with methyl or allyl tweaks, often pop up in modern labs. PF6- anions help shuttle charge without falling apart in harsh conditions, and the piperidinium core resists unwanted side reactions. These properties open doors from safer electrolytes in lithium-ion batteries to platforms for new kinds of sensors. I’ve seen startups using similar compounds to push supercapacitor performance, always balancing chemical toughness with cost.
Stability and Safety: Looking Beyond the Textbooks
Seeing a six-membered ring doesn’t do much good to someone sweating over shelf stability or worried about fumes in a shared workspace. What really hits home from years spent at the lab bench is this: N-Allyl-N-Methylpiperidinium Hexafluorophosphate won’t break down easily. Its structure shrugs off both air and moisture, meaning less time wasted on dry boxes and fewer headaches during storage. The flip side — anytime fluorinated anions enter the picture, waste must be managed responsibly due to potential environmental impacts. Proper disposal isn’t just a rule, it’s a duty. If the industry shifted towards greener engineering, novel approaches could further reduce hazards.
What Could Change Going Forward?
Demand grows for new energy storage materials, and chemists keep tweaking structures for better results. Swapping the methyl or allyl group shifts performance, sometimes in surprising ways. I remember seeing data where one small substitution meant the difference between a stable battery and one that fell apart fast. This tweaking speaks to a broader trend: the journey from academic curiosity to real-world utility requires both precision in the lab and willingness to adjust, whether that’s safer anions or new ways to make these materials cleaner. Balancing these priorities, future research should aim for not just performance, but greener lifecycles from synthesis to disposal.
Game-Changer for Organic Electrochemistry
N-Allyl-N-Methylpiperidinium Hexafluorophosphate—the name’s a mouthful, no doubt. Out in the real world of chemical research, this salt steps up where electrical conductivity matters. Sure, everyone talks about lithium salts for electrolytes, but there’s a strong case for less flashy players. Ask anyone in organic electrochemistry: reliable ionic liquids and supporting electrolytes are always in demand.
This compound finds a spot in that toolbox. When researchers push for efficient, high-yield electrochemical reactions, they look for salts that steer charge flow without side reactions or breakdown. Not every salt keeps up in demanding setups—temperature swings, voltage spikes, solvents that aren’t always friendly. What tips the balance toward N-Allyl-N-Methylpiperidinium Hexafluorophosphate? Scientists like it for its high ionic mobility, its relatively low toxicity compared to some quaternary ammonium salts, and its resilience under harsh reaction conditions.
Building Up Conductive Materials
In the field of material science, this compound gets some attention too. Conductive polymers have changed everything from flexible electronics to biosensors. Crafting those polymers often needs a steady, compatible electrolyte. The hexafluorophosphate anion offers chemical stability, and the piperidinium core helps dissolve in a range of solvents—acetonitrile, propylene carbonate, even some greener picks for labs that care about sustainability.
The right supporting salt influences everything—conductivity, mechanical strength, whole operational window of the polymer. Folks in labs know that a tweak in the supporting electrolyte sometimes brings a leap in performance. Here, the unique structure of N-Allyl-N-Methylpiperidinium boosts solubility and limits unwanted side reactions, which matters for scaling up a research breakthrough into working electronics.
Battery Electrolytes: Carving a Place Amid Heavyweight Contenders
Battery researchers keep hunting for non-traditional electrolytes that dodge some pitfalls of alkali metals. The stability of hexafluorophosphate anion and the asymmetric piperidinium cation bring a tough combination. Not every battery tech needs this kind of salt, but next-gen batteries—especially some experimental sodium-ion or high-voltage prototypes—sometimes benefit from electrolytes that resist breakdown and help extend cycle life.
The market gets flooded with options, and most big-name cell makers won’t touch something unless it’s proven over years. Yet, in labs concentrated on safety and cycle stability, this compound turns up in small-batch tests. Researchers at academic institutions and startup incubators will often swap in N-Allyl-N-Methylpiperidinium Hexafluorophosphate in search of lower flammability and wider electrochemical windows, even if it comes at a cost.
Finding the Sweet Spot Between Performance and Sustainability
Chemicals like this don’t always make headlines, but their behind-the-scenes influence grows every year. Sustainable manufacturing matters more now than ever. Every time labs hit a wall with old-school electrolytes—whether it’s issues with disposal, regulatory hurdles, or unexpected side reactions—innovators start looking at salts like this. There’s always room for better toxicity profiles, more recycling options, and safer by-products.
A few companies now are even exploring custom blends, where N-Allyl-N-Methylpiperidinium Hexafluorophosphate becomes a co-electrolyte, dialing in just the right properties to crack new markets in green chemistry, printable circuits, and wearable electronics. These early adopters understand that true progress doesn’t always come from the biggest breakthrough, but from nudging dozens of small variables toward a safer, more efficient process.
No Magic Bullet, But No Disposable Tool Either
Anyone who’s worked in a chemistry lab knows that utility beats glamour every time. N-Allyl-N-Methylpiperidinium Hexafluorophosphate may not show up in pop science news, but in the places where researchers prize robust electrolytes and durable polymers, it’s already earning its stripes. Pulling together expertise, data, and real-world testing—the game keeps moving forward, one reliable compound at a time.
Getting Practical About Safe Storage
Walking into any laboratory or storeroom, the heavy bottles and powder-filled containers usually sit under a banner of careful order. In my own early days in research, the labels with their hazard signs and expiry dates stuck out far more than the shelves themselves. Storage isn’t just about keeping things tidy; it matters for safety and the power inside each compound.
Take a look at the experience of sodium hydroxide—a popular base, strong but fickle. If the lid slips loose or air seeps in, the reagent reacts with carbon dioxide, forming a crusty, less-effective powder. From the get-go, those who use sodium hydroxide close containers tight and stash them somewhere dry, shielded from light, away from acids. This vigilance isn’t for show. Simple steps like these prevent dangerous reactions and keep the material potent for future experiments.
Why Humidity and Light Make a Difference
Some people think a shelf is just a shelf, but there’s more to it. Humidity creeps in through unsealed windows or leaky containers, and more than one promising experiment has failed because moisture changed the weight or texture of a sensitive sample. Silica gel packs placed in cabinets draw away extra water, protecting substances like anhydrous salts or some pharmaceuticals, which break down just from a humid afternoon.
Photosensitive chemicals demand even more precise attention. Take silver nitrate—exposed to light, it darkens and loses strength. Keeping such materials in amber bottles in dim storage areas ensures they stay useful for much longer. Nobody wants to discover that a bottle has lost its bite purely from sitting near a window.
Temperature Control—More Than Just “Cool and Dry”
So many labels parade “store in a cool, dry place” as guidance, but real practice asks more specifics. Some compounds break down if chilled. Regular refrigerators hold a parade of smells, sometimes with food, introducing risk of cross-contamination. Special flammable cabinets, ventilated and temperature-controlled, sit far away from heat sources. A small difference—such as a room set five degrees hotter than needed—shaves months or even years from a product’s shelf life.
Real stories underline this. In hospital pharmacies, vaccine storage must fluctuate within a narrow two-degree range. During power cuts in my region, generators spring to life. Otherwise, expensive batches go to waste. Digital monitors now watch over temperatures, alerting staff before drugs spoil.
Simple Practices Any Lab Can Follow
Each time a new bottle arrives, mark it with the date of opening. Rotate out older stocks before reaching for something fresh. Proper ventilation keeps vapors from accumulating. Sturdy shelving, labeled clearly, helps staff grab the right compound without reaching over the wrong one.
Training matters. People who understand the risks treat storage as a partnership between discipline and science, respecting both risks and regulations. Inspections by qualified personnel—not just a quick scan—catch problems before they snowball. Chemical manufacturers often print precise instructions, but local experts build on these with hands-on habits suited to their own facilities, climate, and staff.
Clear, well-communicated rules, reliable equipment, and a culture of carefulness work together to minimize accidents and preserve the value of every compound on the shelf.
Chemicals in the Lab Aren’t All Created Equal
Walking through labs over the years, you spot plenty of bottles with long chemical names. N-Allyl-N-Methylpiperidinium Hexafluorophosphate doesn’t catch many headlines, but it carries risks that deserve straight talk. Chemists rely on it for specific reactions, sometimes as an ionic liquid. Just because you don’t see warnings on every bottle doesn’t make something harmless. Overconfidence around new chemicals has caught many researchers off guard.
What Kind of Risks Are Real with This Compound?
Insert this salt into a process, and you’re working with two main pieces: a piperidinium cation and a hexafluorophosphate anion. Based on my own time in organic synthesis labs, hexafluorophosphate raises flags. It’s popular in ionic liquids, yet it’s far from benign. Any salt with this anion brings a risk: in contact with moisture and acids, they sometimes release hydrogen fluoride (HF). This isn’t a minor concern—HF is infamous in certain labs for causing rapid, severe chemical burns that can put a technician in the hospital.
N-Allyl-N-Methylpiperidinium Hexafluorophosphate may not stand out for acute toxicity the way cyanides or some solvents do, but skin and eye irritation remain possible. Synthetic work always exposes people’s hands, forearms, and faces to splash risks. Inhalation can’t be counted out either, especially when fine powders or dust are involved—once, a spill with a similar compound led to breathing issues in a coworker until we aired out the room.
Waste and Disposal Aren’t a Side Note
Disposal practices today demand more attention than a decade ago. Any compound containing hexafluorophosphate should not be poured down the sink. Improper disposal can send PF6– and its breakdown products into water systems, and these fluorinated contaminants don’t break down easily. Some regulatory bodies now flag these chemicals for potential environmental persistence and possible toxic effects on aquatic life.
Respecting Chemicals Means Rethinking Our Habits
Good habits in labs shape safer workplaces. I’ve seen firsthand how complacency builds up. The person "just topping up a flask" without gloves, the fume hood sash left open, or the waste bottle that’s not labelled—the fallout can be real. Material Safety Data Sheets remain a technician’s best friend, even if they seem tedious. Direct experience teaches, too: on a day when an acid spill hit a hexafluorophosphate salt, a smart teammate caught the whiff of HF early, and we got out fast.
Solutions for Reducing Risk
Upgrading safety training should stay on everyone’s agenda. It's not about paranoia—it's about never assuming familiarity equals safety. Push for good chemical inventory management. Working in well-ventilated areas and wearing splash-proof goggles make a difference. Don’t skip gloves, even for a “quick measure.” If an acid spill occurs in the vicinity of these salts, evacuate. Keep calcium gluconate gel on hand, as it helps with HF exposure—better to have it ready before someone needs it.
On a bigger scale, universities and companies ought to reevaluate which chemicals get automatic approval for purchase. Ask tough questions: Is this salt necessary, or can a less hazardous alternative do the job? Too many incidents start because no one asked that simple question.
The Real Value of Purity
Purity forms the backbone of any chemical product, and from my own years of working hands-on with laboratory supplies, I’ve learned that it’s not just a number on a certificate. Purity means the absence or presence of unwanted elements, and it directly changes how a material behaves in the real world. If a product claims 99.9% purity, that margin means more confidence in results, whether you’re doing pharmaceutical research or working with high-precision manufacturing.
This is more important than most people realize. Low-purity chemicals introduce unknown risks—like side products that throw off results, clog reactors, or create unreliable testing conditions. Even small trace contaminants cause headaches. In regulated industries, the difference between 99.5% and 99.9% isn’t a decimal point—it’s a dealbreaker. That’s why quality assurance teams rely heavily on independent batch testing and published certificates of analysis for every purchase.
Packaging Sizes: Meeting Everyday Practicality
You can find packaging sizes to fit almost any job. Most suppliers offer small containers, from 100-gram bottles to 1-kilogram jars, for research and bench chemistry. From my experience, labs go through these at a steady pace. These smaller options cut down waste and lower the risk of contamination since a fresh bottle comes out as needed.
Larger sizes serve production lines and industrial processes. Ten, twenty-five, or even fifty-kilogram drums stack on pallets for bulk orders. Manufacturers who handle daily batches of solvents, acids, or specialty compounds find these bulk packages essential for keeping work flowing. If you’re maintaining a continuous process line, switching to small jars just isn’t practical. It drives up handling costs and creates more containers to clean and dispose of at the end.
Disposable packaging with tamper-evident seals boosts safety by making it easier to spot any issues before use. In the lab, I always trust fresh, properly sealed containers––unopened, clearly labeled, and with expiry dates to back up safe use.
Quality Control and Transparency Matter Most
Manufacturers publish detailed batch records and analysis reports for a reason. Look past flashy marketing and go straight to the data table. Ask for a certificate of analysis with each lot number—see the real impurity profile, not just a stated percentage. Reliable suppliers will never hesitate to share this information.
Then there’s the overlooked detail of shelf life. Purity might hold steady on the day of packaging, but exposure to air, heat, or moisture in storage gradually changes a chemical’s properties. I always store chemicals following best practices, and I keep an eye out for discoloration, unusual smells, or caking—early signs that purity has taken a hit long before the bottle is technically empty.
Building Trust Through E-E-A-T
Everyone wants dependable results, so choosing a supplier with a clear track record makes all the difference. Reputable companies invest in robust testing, transparent documentation, and customer education. Recalling stories from my colleagues, switching to a high-trust brand has reduced downtime, saved money, and prevented costly recalls.
In the end, purity and packaging size link back to real issues: maintaining safety, cutting unnecessary costs, and keeping operations simple. Reliable suppliers empower users with the right packaging for the job and complete transparency about quality—taking much of the stress out of daily operation.