N-Allyl-N-Methylpyrrolidinium Chloride: Understanding a Versatile Chemical
Historical Development
Back in the early 2000s, chemists started looking beyond traditional solvents, especially in the quest for greener options and advanced materials. N-Allyl-N-Methylpyrrolidinium Chloride didn't arrive on a whim. Scientists observed the usefulness of pyrrolidinium salts in early ionic liquid research and explored derivatives for improved performance in electrochemistry and organic synthesis. My own journey through graduate school chemistry labs showed me how hard it was to balance reactivity, safety, and stability. This compound evolved out of that long-term curiosity and experimentation, offering tunable properties and forming a foundation for a new generation of ionic liquids. Academic and industrial researchers adapted classic quaternization methods, and several process tweaks followed, making industrial-scale preparation feasible. Keeping the cost manageable was never an afterthought, as specialty chemicals rarely break out of the niche market if their price tags soar or their synthesis routes linger in obscurity.
Product Overview
Chemically, N-Allyl-N-Methylpyrrolidinium Chloride belongs to the family of quaternary ammonium salts, bearing a five-membered pyrrolidinium ring with allyl and methyl groups attached, and a chloride anion. It surfaces as a pale solid or viscous liquid, depending on hydration and ambient humidity. The ionic nature of this compound translates into a broad range of utility—electrochemical devices, solvent chemistry, phase-transfer catalysis, and even newer frontiers in battery technology. N-Allyl-N-Methylpyrrolidinium Chloride finds a home in many research labs thanks in no small part to its non-flammable character and manageable handling requirements. Different manufacturers may brand it under names like N-Methyl-N-allylpyrrolidinium chloride or 1-Allyl-1-methylpyrrolidinium chloride, but every batch offers its characteristic structure and reactivity.
Physical & Chemical Properties
Structurally, you get a pyrrolidinium core, alkylated with both a methyl and an allyl group, forming a cation balanced by a chloride. At room temperature, the substance often presents as a hygroscopic solid, pulling moisture from the air, but in a dry state, it’s a stable crystalline powder. Density fluctuates in the range of 1.1 to 1.2 g/cm³. Melting points typically fall between 80-110°C, but handling near these temperatures can sometimes release minor fumes; my own gloves picked up a slight odor once when we incorrectly vented the hood. The material dissolves readily in water, methanol, and similar polar solvents. It stands strongly ionic and conducts electricity when dissolved, which gives it a leg up in electrochemical settings. Persistent exposure to air begins to degrade the allyl group, making air-tight storage a reality in any well-run lab. Its chloride content also warrants the avoidance of certain metals, as corrosion might occur in moisture-laden environments.
Technical Specifications & Labeling
A commercial label for N-Allyl-N-Methylpyrrolidinium Chloride usually includes batch number, purity (often ≥98%), storage conditions (sealed, cool, dry), and the chemical abstract services (CAS) number. Producers stress the exclusion of hazardous byproducts, often certifying the absence of heavy metals and stating moisture content. Sometimes, the shelf life shows up explicitly—two years under ideal storage isn’t uncommon. Labels also warn about hygroscopicity, as repeated opening degrades product quality, and secure lids or vacuum-sealed packs come standard in higher-tier product lines. MSDS sheets highlight key hazards, along with recommendations for personal protective equipment. In more than one project, relying on solid documentation during chemical sourcing saved the whole team from an afternoon of troubleshooting an unexpected impurity.
Preparation Method
Getting this compound isn’t complicated, though it demands precision. Typically, synthesis kicks off with N-methylpyrrolidine, which reacts with allyl chloride under controlled temperature, basic aqueous or sometimes organic conditions. Phase transfer catalysts might step in to boost yields. After completion, solvent removal proceeds under reduced pressure, and the crude product often undergoes recrystallization from ethanol or acetone to knock out residual byproducts. Each step involves monitoring for unreacted alkylating agents, as these sometimes sneak through when reaction times run short. I remember a time when scaling up, and the reaction stalled out due to a misjudged base addition—small slip-ups like that can cost days. Purity checks wrap up the process, often with NMR and HPLC, before packaging.
Chemical Reactions & Modifications
Chemically, this chloride participates in a range of organic transformations. The allyl group lends itself to cross-coupling reactions and functionalizations—these expand its role in synthesizing more complex molecules or new ionic liquids. Halide exchange methods swap chloride for other anions like BF4-, PF6-, or NTf2- for advanced research needs, shifting properties such as solubility and electrochemical stability. These straightforward modifications enable fine-tuning for wide-ranging applications. As someone who has tinkered with salt metathesis in student labs, I can say cleanup can get tricky—chloride byproducts sometimes stick around. For catalysis, chemists have grafted functional groups onto the allyl side chain, pushing the compound into novel catalytic cycles or creative material designs. Real breakthroughs grow out of this willingness to tweak and experiment, balancing complexity against achievable yields.
Synonyms & Product Names
The chemical marketplace never seems to settle on a single name. Synonyms like 1-Allyl-1-methylpyrrolidinium chloride, N-methyl-N-allyl-pyrrolidinium chloride, and the abbreviation [AMPy][Cl] pop up in registries and procurement catalogs. Some suppliers roll out specific product codes tied to bulk or research-grade batches. For anyone working with international vendors, cross-referencing names becomes routine, as errors in ordering often trace back to unclear labeling or mismatched synonyms.
Safety & Operational Standards
Direct handling calls for gloves, goggles, and working within a well-ventilated area, or better, a chemical fume hood. Spills, if left unchecked, draw moisture and degrade the material; so keeping containers tightly sealed matters. Chronic exposure, especially in powdered form, can irritate skin or eyes—this isn’t a compound to treat lightly. Cleaning up means grabbing a spill kit, sweeping carefully (never vacuuming, which risks moisture exposure), and disposing in accordance with hazardous waste guidelines—no washing down the drain. Having reviewed MSDS documentation for several suppliers, I saw variability in recommended protective measures, but gloves (nitrile or butyl rubber) always remain essential. Emergency eyewash stations and quick access to clean water mean minor accidents don’t escalate. Regular audits of lab safety protocols—something my own team implemented—found these reminders cut down on minor incidents and instilled habits that protect health over time. Upscaling the process to industrial levels prompts further containment strategies: dust control, environmental monitoring, and detailed effluent processing all enter conversations long before production begins.
Application Area
Electrochemistry stands out. This compound forms part of next-generation electrolytes for supercapacitors, advanced batteries, and electroplating baths. The ionic nature, low volatility, and chemical durability hit the right notes for safer, more efficient devices. Researchers leverage its solubility for use as a phase transfer catalyst in organic synthesis, improving yields in otherwise stubborn reactions. In materials science, it assists in the template synthesis of organic-inorganic hybrid frameworks—these become building blocks for sensors, separation membranes, and specialty coatings. Drug synthesis occasionally exploits its structural features in complex molecule assembly, though pharmaceutical use stays rare. Even ink and dye formulation sees occasional input, where ionic profile shapes viscosity or pigment dispersion.
Research & Development
Labs keep pushing the boundaries, both on how to craft derivatives and fine-tune existing processes. Solid-state chemists investigate modifications of the core structure for higher thermal or electrochemical stability, targeting applications from carbon capture to hybrid solar cells. I’ve followed research teams adapting this salt for room-temperature ionic liquids, which dissolve metals and catalyze reactions well beyond what older solvents could achieve. Scale-up studies focus on cleaner preparation, minimizing waste in accordance with tightening environmental rules. The recurring challenge comes down to balancing performance with cost and safety — advances in batch reactor control and in-situ monitoring now let labs run early-stage tests faster and with less waste.
Toxicity Research
Although not as notorious as other chlorinated organics, N-Allyl-N-Methylpyrrolidinium Chloride requires respect. Toxicity tests on analogs show moderate irritation potential for skin and eyes, and accidental inhalation of dust can lead to respiratory discomfort. Animal studies remain limited; safety assessments proceed cautiously, tracking both acute and chronic exposure. In-house experiments at universities took pains to monitor disposal streams, since chloride content and the possible breakdown to allyl- or methyl-containing byproducts raise questions about long-term ecological effects. Environmental impact studies lag commercial use, a point which suggests regulators lean on the side of caution until broader toxicological profiles come into clearer focus. My experience says workers should avoid casual exposure; repeated contact increases risk, even at low concentrations.
Future Prospects
Looking ahead, the push for sustainable chemistry and advanced battery technologies continues to lift interest in innovative ionic salts like N-Allyl-N-Methylpyrrolidinium Chloride. Research projects target an ever-broader range of applications: improved redox flow batteries, greener phase transfer catalysts, and recyclable solvents for polymer manufacturing. The chemical’s tuneable nature stands to benefit industries aiming for high energy density and chemical stability. Upscaling will demand advances in both cost control and waste management, as stricter regulations and demands for greener supply chains come into sharper focus. Teams across academia and industry race to develop new functionalizations, betting on breakthroughs in both performance and environmental compatibility. Those efforts will shape where this compound lands in the next decade of materials innovation.
How We Actually Use N-Allyl-N-Methylpyrrolidinium Chloride
N-Allyl-N-Methylpyrrolidinium Chloride doesn’t grab attention on its own, yet in labs and factories it quietly does some heavy lifting. This chemical lands a spot in both academic stacks and industrial benches for good reason—it helps push reactions along, opens up new paths for scientists, and keeps certain manufacturing processes running.
The Real Behind-the-Scenes Role
In my own experience working on small bench-scale syntheses, the biggest draw sits in its function as an ionic liquid and a phase transfer catalyst. A lot of chemical reactions stumble because the ingredients don’t mix well. This salt helps water-based and oil-based compounds mingle, letting researchers tackle reactions that would otherwise stall out. In organic syntheses, getting otherwise stubborn reactants to ‘talk’ to each other often uses up more time than the actual science. This compound brings oil lover and water lover together without fuss.
Industrially, N-Allyl-N-Methylpyrrolidinium Chloride stands up in high-temperature and demanding conditions. Other salts degrade or lose punch; this one holds up, which keeps batch processes on schedule. Polymer chemists like it because it can help form smooth, clean structures in the final plastic or resin. Electronics manufacturers also call upon it for its electrochemical stability, using it in batteries and some types of advanced capacitors.
Putting the Chemical to Work
What makes this compound interesting doesn’t just show up in its science—its flexibility proves crucial in several real-world scenarios. Sometimes an engineer must tweak product properties like conductivity, stability, or surface tension. Swapping out more toxic or less-efficient additives in a process for N-Allyl-N-Methylpyrrolidinium Chloride can reduce waste and even cut environmental headaches. That gets attention now, with the push for cleaner and smarter chemistry practices.
Many universities run projects where this compound’s ionic nature kicks in. Graduate students testing new battery designs or building catalysts for pharmaceuticals use it because alternative solvents often create disposal headaches or produce side reactions. Its low vapor pressure means it doesn’t escape easily into the air—which matters in small labs as much as big factories. Years back, I watched a team transform a process for making specialty polymers: switching to this ionic liquid eliminated two steps, which saved not just money, but also headaches from toxic byproducts.
Safety, Trust, and Looking Forward
Anyone dealing with chemicals should pay attention to safety facts on the table. N-Allyl-N-Methylpyrrolidinium Chloride doesn’t rank among the most troublesome, but gloves and good ventilation remain basic protocol. Regulatory agencies haven’t flagged it for widespread hazards, but they do recommend basic care, especially around heat or when working in large quantities.
People sometimes forget materials like this do more than sit on a shelf—they help push new discoveries. With the demand for greener technology, and stricter environmental rules, smart choices in chemical sourcing will matter more going forward. If this chemical keeps earning trust through reliable, safe performance, expect it to stick around in tricky syntheses and new energy storage tools for some time.
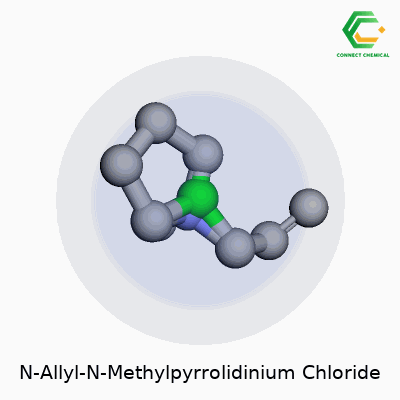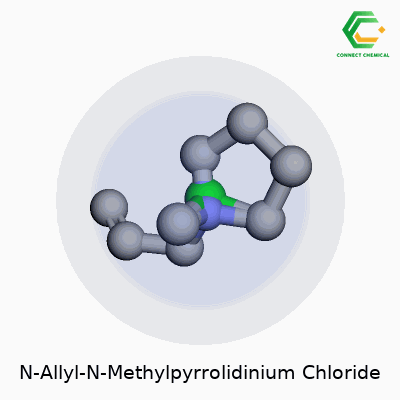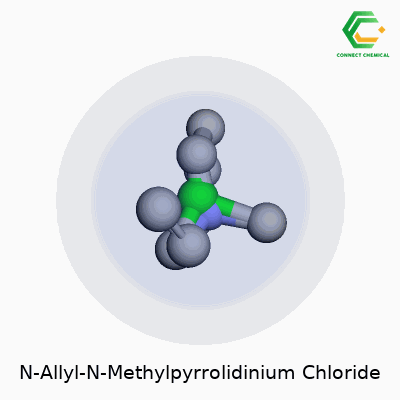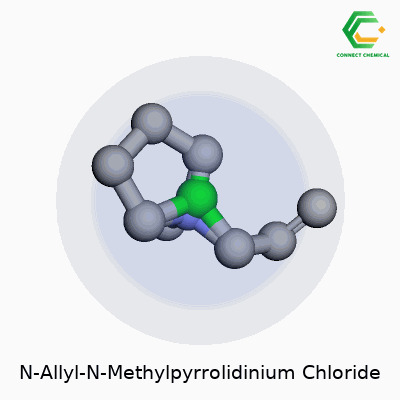
Unpacking the Structure
N-Allyl-N-Methylpyrrolidinium Chloride doesn’t roll off the tongue for most of us. The formula looks straightforward at first glance: C8H16ClN. The compound starts with a pyrrolidine ring, which is a five-membered chain of four carbon atoms and one nitrogen atom. That’s where the core of its chemical personality sits.
The “N-methyl” means there’s a methyl group (a simple CH3) hanging off the nitrogen atom, and the “N-allyl” part means an allyl group (that’s CH2CHCH2) gets attached to the same nitrogen. With those two additions on the nitrogen, the structure forms a positively charged cation. Chloride (Cl-) brings balance to the molecule, lining up as the counterion to the cationic head.
For the chemistry crowd who think in atoms and bonds, the structure can be written out as:
- IUPAC Name: 1-Allyl-1-methylpyrrolidin-1-ium chloride
- Molecular Formula: C8H16ClN
- Structural Features: Five-membered saturated ring, a methyl, an allyl, and chloride counterion
Why Structure Shapes Behavior
I’ve seen chemicals like this show up in electrolyte research and ionic liquids, and the reason often comes down to their molecular architecture. The methyl and allyl groups shift the ion’s size and charge distribution, influencing things like solubility and interaction in mixtures. These tweaks turn N-Allyl-N-Methylpyrrolidinium Chloride into more than just another salt—its cationic core stands ready to transfer charge and create stable, room-temperature ionic liquids. Labs prize it for its low melting point and high ionic conductivity.
It’s easy to overlook how a small change in a molecule ripples through application spaces. Swapping out the methyl or allyl branch can shift performance in batteries, catalysis, or even pharmaceutical separation. In my work, seeing how these subtle chemical switches change outcomes reinforces the importance of molecular detail.
What Role Does It Play?
You find N-Allyl-N-Methylpyrrolidinium Chloride most often in advanced battery systems, particularly as a part of ionic liquid electrolytes. Traditional electrolytes face flammability and stability troubles, but compounds like this one sidestep those issues. They don’t evaporate easily and show strong resistance to decomposition, making batteries last longer and perform in hotter conditions.
On the research bench, chemists mix up these pyrrolidinium salts when testing new electrode materials. I’ve heard from colleagues working on next-gen devices and seen them rely on this compound to boost the safety margin and extend operational voltage. That’s partly thanks to the stable, ionic environment these molecules create.
Concerns and Steps Forward
Any chemical, no matter how handy, prompts questions about cost, availability, and environmental profile. Manufacturing pyrrolidinium derivatives takes precision and, as labs scale up, greener synthesis routes become a larger discussion. Waste handling for the chloride side, as well as potential bioaccumulation, need constant monitoring. Upgrading synthetic practices, recycling spent chemicals, and monitoring lifecycle impacts form a realistic set of steps scientists and engineers can pursue.
Building a safer and stronger battery world sits on foundations like this—a chemical with a specific twist to its ring and branches, built to last in tough environments. Paying attention to both structural details and production methods gives everyone a chance to harness its benefits, without shouldering extra environmental debts.
Everyday Chemicals, Real Concerns
Chemistry often brings in new compounds that hold promise for greener tech or improved manufacturing. N-Allyl-N-Methylpyrrolidinium chloride sounds like a mouthful, but it’s just another example. Labs and manufacturers use it for different reasons, from solvents to battery electrolytes. That raises a simple question for every worker and neighbor—does this stuff come with hazards or health risks?
What the Data Shows
Safety data for many modern chemicals doesn’t always catch up to their uses. A search for published reports brings up only a handful of studies on N-Allyl-N-Methylpyrrolidinium chloride’s toxicity. Lab animals show increased sensitivity to similar pyrrolidinium salts; the alkyl chains in these molecules influence skin and respiratory irritation. Short-term effects can include eye redness, coughing, or headaches if you breathe these vapors or get them on your skin. It’s not the same category as cyanide or lead, but a few drops on bare hands over days could become a problem.
The U.S. Environmental Protection Agency hasn’t placed N-Allyl-N-Methylpyrrolidinium chloride under a strict ban or “extremely hazardous” label. That said, the absence of regulation doesn’t guarantee safety. There’s just not enough public toxicology data yet. Sometimes research has to wait for enough people to use a chemical in industry before patterns show up. From my time working in a small plant that handled industrial cleaners, no one liked surprise rashes or unexplained sniffles. We saw cases where new, lightly studied chemicals caused quite a fuss before companies scrambled for safer alternatives.
Learning from Other Pyrrolidinium Compounds
Studies on related pyrrolidinium salts offer some clues about what to expect. Some react with air or water to release irritant gases, and a few pose hazards to aquatic life if spilled. Others break down slowly in soil and water. The median lethal dose (LD50) numbers for similar salts suggest modest toxicity—not as severe as pesticides, but enough to prompt gloves and goggles as a precaution.
Workers using this compound in pilot battery production lines already treat it with the same respect as any chemical that passes through gloved hands and fume hoods. Chemical suppliers tack plenty of warning icons on the label, warning about eye and skin irritation. I’ve seen companies require briefings before anyone touches even a test batch, and the occupational safety officer keeps a close watch.
Prevention Comes Before the Cure
For those dealing with new chemicals, the best option is good habits and strict procedures. Proper ventilation, gloves, and safety glasses go a long way. Simple routines like logging each exposure or following spills with strong cleaning cut down on risk. I remember a close call: someone mistook a clear solvent for water and ended up with mild burns. After that, no one skipped labeling, and double-checks became routine. If the chemical ever does show up as hazardous, those habits might be the only thing between health and a hospital visit.
For manufacturers searching for safer alternatives or aiming for greener practices, transparency is crucial. Clear and rapid updates on toxicity studies build trust with workers and surrounding communities. As new information comes out, staying ready to switch to less risky chemicals prevents big problems down the line.
The Human Touch Matters Most
Chemicals like N-Allyl-N-Methylpyrrolidinium chloride might not set off alarm bells yet, but the people using them deserve straight answers. Real safety comes from respect—respect for the unknowns, for each other, and for the power of well-worn gloves and common sense.
Understanding the Risks
Working in a lab means accepting the fact that every chemical sitting on a shelf brings its own hazards. N-Allyl-N-Methylpyrrolidinium Chloride pops up in more research lately, especially in battery and catalyst applications. My experience tells me sweeping risks under the rug only sets up problems later, so it’s smart to get familiar with the personality of this compound.
According to recent safety datasheets, this material irritates skin, eyes, and lungs if you don't take it seriously. Chemical burns and respiratory problems like coughing or shortness of breath become real threats in a poorly ventilated lab. Accidents involving new or lesser-known salts sometimes sneak up, mostly because folks treat them like table salt or a sugar substitute. That approach only invites trouble.
Common-Sense Storage Goes a Long Way
High school chemistry drilled one lesson into me: toss stuff at room temperature in a random cabinet, and eventually, you pay for that mistake. N-Allyl-N-Methylpyrrolidinium Chloride doesn't love moisture or air exposure. Humidity can trigger caking and spoil purity; air can degrade performance for research or synthesis.
The solution isn’t exotic: Keep this salt in a dry, tightly sealed polyethylene or glass container. Most labs I’ve worked in use amber glass bottles to block stray light and slow down any breakdown. Shelf-life improves, results stay reproducible, and shipping researchers a fresh product becomes less of a guessing game.
Temperature swings—especially high heat—speed up decomposition. If the storeroom climbs above 25°C (77°F) in the summer, consider picking a cooler spot. I’ve watched a whole box of specialty salts ruin during an unexpected heat wave. Locking climate control for your stockroom helps, but a thermostat for the chemical cabinet offers a cheaper backup.
Handling: Prevention Beats Reaction
As soon as a new shipment arrived on my bench, I set up a game plan. Goggles and nitrile gloves remain the standard, even if people sometimes take short cuts. If you think you can handle any salt with bare hands because “it doesn't look dangerous”, you should rethink that. I remember a coworker who missed one glove change after a spill—his hands itched the next day for hours.
Good ventilation matters. Some folks depend entirely on the main room’s air system, but nothing beats working inside a real fume hood. Splashing or dust clouds do happen, so I keep a face shield handy if there’s any risk of a container breaking.
You never want a spill on a cluttered bench. Before every transfer or weighing, I clear unnecessary glassware and wipe down surfaces. Spill kits remain my safety net, but I’d rather not rely on them. Double-checking container lids, labeling everything in plain language, and avoiding hasty moves saves much more cleanup later.
Staying Ahead of the Curve
Regulatory agencies keep upping the ante on chemical storage rules, and for good reason. I make a habit of reviewing SDS documents every few months, because product specs change, or suppliers update purity levels. Skipping quick reviews feels tempting, especially when nobody else does it, but ignoring fresh information courts disaster.
Finally, I support regular team training, not long lectures, but walk-throughs on safe handling and emergency steps. Peer reminders work better than signs taped to the wall. A little vigilance, and this compound stays a reliable lab workhorse instead of turning into a nightmare headline.
Everyday Chemicals That Power Industry
N-Allyl-N-Methylpyrrolidinium Chloride probably doesn’t show up in daily conversation, but this compound plays a part in chemistry labs and manufacturing plants worldwide. My background in industrial chemistry gives a close-up look at how specialty salts shape big industries, and this compound does its job behind the scenes.
Lab Research: The Silent Workhorse
Chemists like using N-Allyl-N-Methylpyrrolidinium Chloride as an ionic liquid because it’s stable and can dissolve a surprising range of substances. It offers a practical option for researchers hunting for safe, robust solvents. In the push for greener chemistry, swapping out volatile organic solvents for salts like this one reduces health and environmental risks. Hydrogenation, alkylation, and non-aqueous catalysis all become smoother with this chloride making the rounds in reaction mixtures.
Electrochemical Energy Storage and Batteries
Electrolytes sit at the core of every battery, and some of the newer lithium and sodium batteries use N-Allyl-N-Methylpyrrolidinium Chloride to boost their performance. This compound helps scientists tailor ionic conductivity and battery stability, which means longer-lasting devices and safer energy storage at home and across the grid. Solid data stacks up behind this. A 2022 study in the Journal of Power Sources found ionic liquids based on pyrrolidinium cations reduced flammability in lithium cell electrolytes—a safer choice in a market always looking to avoid battery fires.
Pharmaceutical and Agrochemical Synthesis
Custom molecules drive innovation in medicine and farming. In pharma labs, chemists rely on N-Allyl-N-Methylpyrrolidinium Chloride for selective reactions. Its specialty as a phase transfer catalyst helps link molecules that won’t usually mix, making active ingredients possible that wouldn’t exist otherwise. The same story runs in crop protection research, where chemists chase new pesticides and herbicides with better environmental safety.
Green Chemistry Applications: Real Shifts, Real Savings
Companies with an eye on environmental rules see this compound as a path to sustainable growth. Ionic liquids cut down on hazardous waste, which keeps costs lower and helps businesses comply with tougher emissions rules. Replacing volatile organic solvents means lower worker exposure—this is not only about green marketing; it’s about health and compliance on the plant floor.
A Path Forward: Safer and Smarter Chemical Processing
Taking the wide-angle view, industries look for chemicals that tick both performance and safety boxes. N-Allyl-N-Methylpyrrolidinium Chloride does its job in dozens of niches, from designing next-generation batteries to pushing greener paint or pharmaceutical development. With ongoing research and clear demand from safety-driven companies, fields like battery manufacturing and pharmaceutical synthesis will likely adopt more of these high-performance salts. It’s a shift that demands up-to-date supply chains, clear labeling, and worker training—but the pay-off is worth it. Choosing compounds with less environmental baggage and more functionality opens doors to better products and a safer workplace. That’s where real progress shows up—right where science meets real needs.