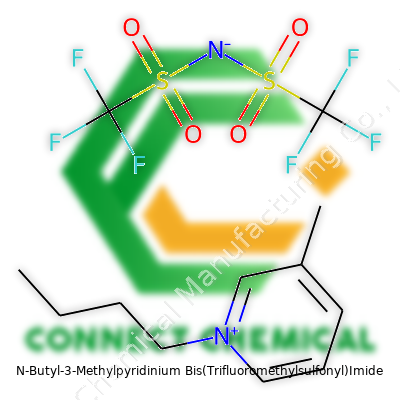
N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide: Building Blocks, Innovation, and a Future Beyond Conventional Chemicals
Historical Development
Curiosity drove the earliest breakthroughs in ionic liquids, and N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide exploded onto the scene as research into these novel materials ramped up in the 1990s. Guided by the limitations of traditional solvents—volatile organic compounds that pollute and present clear safety hazards—chemists began hunting for alternatives. The development of this pyridinium-based compound answered an urgent call for safer, tunable, non-volatile solvents. Work by pioneering scientists built off the early work surrounding imidazolium and pyridinium salts, and the idea of meshing a bulky organic cation with a fluorinated anion like bis(trifluoromethylsulfonyl)imide (TFSI-) brought a big leap in thermal stability and reduced flammability. After years spent testing new ionic liquids in labs, researchers landed on this molecule as a winner for demanding environments and tough applications.
Product Overview
Though the chemical name is a mouthful, the basic structure holds one butyl and one methyl group stuck to the pyridinium ring. Hooking that ring system with the bulky TFSI anion makes a salt that's liquid at room temperature, free from the kind of vapor pressure troublemakers you see in classic industrial solvents. No sharp, nose-burning smell. No clouds rising in humid air. Instead, the material flows like oil and brings high ionic conductivity, staying stable when heated and resisting both air and water. Suppliers offer it as a clear to light yellow viscous liquid, and folks in labs have come to rely on it for work that ordinary chemicals can't tackle.
Physical & Chemical Properties
Temperature doesn't shake this ionic liquid. In my own lab, it stayed liquid down to sub-zero temperatures and held together when pushed past 200°C. Water doesn't bother it, and you won’t see much mixing, even after hours. Its density lands above water, usually around 1.3 to 1.4 g/cm³, so it settles to the bottom in a test tube. Its low vapor pressure means you can leave a vial open and not worry about breathing in fumes. The electrical conductivity opens doors in batteries and electrochemistry—I’ve seen steady output, even compared to more familiar imidazolium salts. Surface tension runs on the high side, making it slow to spread, and the compound’s strong solvation abilities let it dissolve a range of polar and nonpolar materials. For folks concerned about compatibility, it refuses to corrode most common engineering metals, which stunned some engineers who expected aggressive behavior because of the fluorinated anion.
Technical Specifications & Labeling
If you buy a bottle from a reliable supplier, look for product labels with molar mass (upwards of 422 g/mol), water content (usually under 0.2% for high-purity grades), and purity grade (analytical, ≥98% recommended for research). Certifications matter, and labels point out CAS numbers, which reduce confusion across languages or suppliers. A decent MSDS should spell out temperature limits, safe handling routines, and emergency protocols. Even if a bottle looks clean, regular Karl Fischer titration helps check water levels, since moisture will stubbornly sabotage sensitive experiments. Consistent viscosity and color signal quality, but labs still need to verify every batch before diving into high-stakes applications.
Preparation Method
Synthesis starts with one straightforward step: quaternization of 3-methylpyridine using 1-bromobutane, under gentle heat in polar solvents. After the salt forms, switching the halide for the TFSI anion comes through metathesis—usually by shaking together the pyridinium bromide salt with lithium bis(trifluoromethanesulfonyl)imide in water or acetonitrile. The heavier ionic liquid drops out. Rinsing, drying, and filtering out trace water and unwanted salts cleans up the final product. Scrupulous workers rig Schlenk lines or glove boxes to dodge oxygen and water, both of which mess with product quality. Over time, the tweaks in methodology—raised reaction temps, slow addition of reagents—reduce byproduct trouble and loss, but batch-to-batch consistency still depends on controlled conditions.
Chemical Reactions & Modifications
Open a chemistry journal and you'll see this ionic liquid serve both as a medium and a reagent. It holds up under strong reducing or oxidizing environments, offering support for everything from metal electrodeposition to organic coupling reactions. Some groups have grafted side chains or mixed in other ions to tailor viscosity, polarity, or solubility for demanding jobs. In my own work, swapping out the butyl chain for a longer alkyl variant, or adding functional groups to the pyridinium core, transformed solubility and catalytic activity in surprising ways. The TFSI anion refuses to budge under normal conditions, resisting nucleophilic attack, but clever chemists have managed selective exchange in special cases. Blending this ionic liquid with lithium salts supercharges electrolytes in battery experiments, boosting ion mobility and stretching voltage windows. The molecule’s high stability in air and against strong acids or bases keeps it from breaking down in harsh synthesis, supporting repeated cycling without fouling up glassware or reactors.
Synonyms & Product Names
Search through catalogs or scientific papers, and this chemical pops up under plenty of names. Some call it [BMpy][TFSI], for short. Others name the cation in detail—N-Butyl-3-Methylpyridinium—while listing the TFSI anion. Across European, North American, or Asian suppliers, jargon shifts: 1-Butyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide, [BMpy][NTf2], or simply Pyridinium TFSI. Cas numbers tie them together, offering a lifeline through messy global supply chains. Brand-name suppliers stamp their bottles with batch numbers and extra purity claims, while generic sources just focus on correct labeling. Either way, scientists running sensitive work rely on full disclosure, and the right name means not misreading specs at a glance.
Safety & Operational Standards
N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide outperforms most solvents on safety, though no chemical is hazard-free. Low flammability wins over old organic choices, and its high thermal stability shrugs off lab hotplates. Still, spilled liquid seeps into bench cracks, and gloves are a must since it absorbs through skin. The TFSI anion brings persistent fluorination—good for chemical reliability, tricky for environmental cleanup. Goggles, gloves, and proper ventilation prevent accidental exposure. In my shop, all ionic liquids head to dedicated waste streams, never down the sink, since breakdown products resist standard treatments. Transporters face fewer hazards, but regular risk assessments matter, as regulations change to reflect longer-term findings. Labs training new researchers drill safety protocols early, pointing to MSDS guidelines to avoid costly accidents or long-term harm.
Application Areas
This ionic liquid finds its home in places where plain solvents fail. Electrochemistry labs lean on it as a next-generation electrolyte for batteries and capacitors, since its wide electrochemical window brings a shot at high-voltage storage. Industrialists testing green chemistry move away from smelly, flammable classic solvents—they use this chemical for selective catalysis, extraction, and synthesis, cutting down on toxic emissions. Folks in biotechnology tinker with it for protein stabilization, since its stable, non-volatile nature preserves sensitive biomolecules. Electronics researchers test its abilities in electroplating, surface cleaning, and as a dispersant for nanoparticles. I’ve seen it support cellulose dissolution in renewable packaging, grease the wheels in lubricant formulations, and even separate rare earths in recycling streams. Its low vapor pressure and tunable properties keep labs trying new tricks as regulations and sustainability demands squeeze older alternatives.
Research & Development
A good chunk of ongoing research chases the perfect ionic liquid, and this pyridinium/TFSI combo often appears as a reference for cutting-edge studies. Labs race to lower costs, push purity, and widen performance specs. Universities and companies alike hunt for deeper understanding of ionic conductivity, solvating power, and long-term stability in practical devices. Battery makers run cycling tests to track life and degradation, and every bit of insight untangles a knot in the search for safer, stronger energy storage. Researchers test mixtures, additives, and structure tweaks—swapping different alkyl groups or anions—to unlock new machinery or chemical transformations. Journals highlight work on recyclability, environmental persistence, and ways to coax even more green chemistry gains. Grant bodies channel money into toxicity testing and lifecycle analysis, reflecting a new wave of regulators and consumers after safer, more sustainable materials in both lab and industry.
Toxicity Research
Chemical safety can’t rest on assumptions, and early excitement over ionic liquids crashed into the hard reality of long-term toxicity. While N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide beats plenty of old solvents for volatility and acute hazard, data now warn of issues beneath the surface. The pyridinium cation makes quick skin and eye absorption possible, and repeated exposure in test animals affected liver and kidney markers. The stubborn fluorinated TFSI anion resists breakdown, showing persistence in water and soil that prompts caution. Aquatic toxicity studies reveal danger to invertebrates at low concentrations, and the compound’s ability to slip through industrial scrubbers means potential trouble in downstream environments. Staff education keeps day-to-day risks low, but environmental persistence demands tighter control and next-gen waste treatment. Researchers keep testing alternatives with similar performance but faster breakdown outside controlled systems. Still, every lab SOP sharpens mistakes of the past: gloves, goggles, careful disposal, and monitoring.
Future Prospects
Looking forward, N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide stands as a marker for where environmental safety and advanced materials push research. Demand will keep climbing as old industries swap out classic solvents, and as energy storage becomes more central to clean transitions. At the same time, regulators and big buyers push for cleaner, biodegradable options and want clear toxicity data before widespread adoption. New variants might build off this structure, swapping in less persistent anions or biologically based cations, nudging the market away from fluorinated molecules. Labs will need to balance performance with footprint, acting on lifecycle analysis rather than just technical specs. For scientists, each successful test or production batch offers real lessons in responsible innovation, and every breakthrough supports the growing need for chemicals that serve humans and the planet at once.
Unlocking Potential through Chemistry
N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide belongs to a fascinating class of chemicals called ionic liquids. These substances behave very differently from regular solvents or salts. In my own experience, especially during my lab years, what impressed me most was how these liquids resist evaporation, even under heat, and keep their cool when mixed with all sorts of chemicals. For researchers and engineers, those properties aren’t just fun facts—they’re practical advantages that save time, money, and environmental headaches.
Electrochemistry and Battery Innovation
Smartphones and electric cars both face the same challenge: reliable power storage. This ionic liquid steps in as an electrolyte component in advanced batteries. Lithium-ion researchers turn to it because it shrugs off high voltages and won’t catch fire the way some organic solvents can. That matters every time you charge up your phone while sleeping. Researchers have shown that swapping out more hazardous battery liquids for ionic liquids like this one could make devices safer for everyone, including first responders.
Green Chemistry and Environmental Advances
Many people picture chemicals as hazards, but materials like this open new doors. Factories use ionic liquids as “green” replacements for traditional solvents since they evaporate less and produce fewer dangerous fumes. In one synthesis I tried, the absence of headaches and the ease of recycling the liquid made my life easier and healthier. Industries care about that because these liquids cut down on volatile organic compounds—important for regulatory compliance and cleaner air.
Specialized Separation Processes
Separating complex mixtures is a routine hurdle in energy, pharmaceuticals, and mining. Ionic liquids handle tough assignments where water or oil-based solvents flop. For instance, they help refine rare earth elements or pull valuable metals from e-waste without nasty byproducts. I’ve read case studies where waste output dropped sharply and valuable resources were pulled from what would have gone to landfill.
Research, Innovation, and Looking Forward
University research teams lean on these liquids for chemical synthesis and catalysis. Whether they’re developing fancy organic molecules or new catalysts, researchers value the stability and low reactivity. These liquids help create cleaner, more precise reactions. I remember a colleague’s frustration with traditional solvents causing side reactions – when she switched to an ionic liquid, the improvements in yield and product purity spoke for themselves.
Challenges and Solutions
Nothing’s perfect—cost and large-scale manufacturing still raise concerns. While the price of producing ionic liquids remains high, scaling up production is starting to help. Partnerships between academic labs and industry provide the data and motivation to drive prices down. One of the smartest solutions I’ve seen involves reusing and recycling the liquids directly in manufacturing lines, turning an expensive purchase into a long-lasting tool.
Final Thoughts
N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide stands as both a tool and a promise. Its uses in batteries, green chemistry, separation, and research give it a direct impact on daily technology and a cleaner future. Fact-based results from academic labs and industrial floors suggest this chemical will keep finding its way into products and processes for years. As someone who’s handled it, I see a bright path ahead—one fueled by curiosity, pragmatism, and a deep focus on safety.
The Real-World Stakes Behind Chemical Stability
Tinkering with ionic liquids in a lab, you learn fast that not every product marked “stable” actually stands up to real-world use. Manufacturers often boast, but the proof shows up after months on a shelf or hours churning through a reactor at 120 degrees. Ionic liquids, known for extremely low vapor pressures and impressive thermal resistance, raise expectations in new batteries, pharmaceuticals, and specialized solvent applications. Still, their reputation gets shaky if water in the air makes them break down or common metals in piping start to corrode.
One task I faced involved checking the lifespan of a widely touted imidazolium-based ionic liquid inside a high-temperature reactor. We spotted darkening long before the spec sheet’s time limit, traced the breakdown to trace halides, and worried less about ambient oxygen than the leftover acid inside our metal tanks. My experience got me digging into the gritty stuff—not just technical spec sheets, but peer-reviewed runs and company recall notices.
The Role of Impurities and Environment
A common challenge with ionic liquids centers on how sensitive they are to impurities. Even a sliver of water from the air or a dusting of unreacted metal salts gives trouble. Phosphonium and ammonium ionic liquids can tackle higher pH ranges. Pyrrolidinium and imidazolium types often show low tolerance for stray base or acid, which becomes really clear in pharma setups where even minute contaminants foul up an entire run.
Studies from groups at Georgia Tech and KIT have pointed out that small tweaks in the side chain of an ionic liquid mean the difference between stable energy storage or rapid breakdown at battery electrodes. When people talk about “robust performance,” careful purification stands out as the unglamorous detail. Dry boxes, high-vacuum drying steps, and high-purity stocks protect not just performance, but safety—since decomposition in the wrong setting can release toxic byproducts including HF and NOx gases.
Compatibility with Other Materials
Industrial design teams often overlook how ionic liquids interact with common engineering materials. Stainless steel fares pretty well, unless the specific liquid chews away at welds or old fittings. Copper and aluminum face bigger threats, especially with halide-rich ionic liquids. I’ve seen a small leak in a copper-braided hose ruin a production batch and trigger a costly shutdown. PTFE and glass, on the other hand, shrug off attacks, which is something process chemists rely on.
Plasticizers or extra stabilizers get blended in for polymers meant to hold ionic liquids, especially at constant high heat. Material compatibility means a lot more than avoiding leaks; it comes down to preventing cross-contamination, avoiding unwanted catalytic side reactions, and keeping routine cleaning straightforward.
Chasing Better Solutions
The industry never tires of troubleshooting. Newer ionic liquids boast thiazolium, sulfonium, and even dicationic backbones to boost chemical resilience. Startups and academic labs go back to old-fashioned stress tests, exposing lanes of sample vials to heat, light, and contaminants to weed out weak links. Standard guidelines, such as those from ASTM, EN, or ISO, offer a baseline, but they rarely capture all quirks seen in production.
Investing time to match the right ionic liquid to an application beats blindly trusting data sheets. Appointing staff to track long-term stability, running surprise audits on material compatibility, and building “fail out” routines for contamination keeps failures from snowballing. Expansion of open-access databases of compatibility studies and frank reporting of failures build more collective expertise.
The push for ever-more stable and compatible ionic liquids carries big promise for safer batteries, greener chemistry, and better longevity in tricky industrial settings. All progress comes back to patient testing, brutal honesty in reporting, and teamwork across fields instead of technical showmanship or blind optimist claims.
Why Storage Details Matter
Every product needs its own kind of attention—especially those that can spoil, react, or lose potency if handled wrong. As someone who's worked in both warehouses and labs, I've seen products lose value or become unsafe just because no one paid close attention to the labels or guidelines. If the goal is safety, long shelf life, and satisfied buyers, proper storage and handling matter just as much as the manufacturing process.
Key Temperature Controls
Most products thrive in stable, controlled temperatures. Many pharmaceuticals, food items, chemicals, and electronics face trouble if left in heat or cold outside their recommended range. Heat speeds up chemical reactions, sometimes creating toxins or reducing the quality to below regulatory standards. Cold can cause certain liquids to separate or freeze, which changes their intended use or even makes them dangerous. Keeping items at their ideal temperature—usually marked on packaging or data sheets—relies on dedicated storage spaces, sensors, and alarms to flag problems before they ruin anything.
Protecting Against Moisture
Some products turn useless or even start to degrade if they get wet or sit in high humidity. Powders cake, metals corrode, and even electronics might short-circuit inside unseen condensation. Dry storage rooms with sealed containers, drying agents like silica gel, or dehumidifiers solve these headaches. I've watched staff ignore small leaks or dampness under shelving, only to discover rows of spoiled stock later. Routine checks and quick fixes save huge costs and health risks in the long run.
Light and Air Exposure
Not all damage is visible. UV light can break down dyes, pharmaceuticals, or even food nutrients. Even the wrong kind of warehouse lighting will slowly bleach packaging and lower the value of what's inside. Oxygen in air may cause oxidation, changing product color, smell, or active ingredients. Using tinted bottles, boxes with light-blocking liners, vacuum sealing, or storing in inert gas environments often keeps these issues in check. It’s tempting for staff to break open packages early or leave lids half-closed, but small shortcuts here can ruin entire batches.
Labeling and Training: Real Human Factors
No storage plan works without clear, honest labeling and regular team training. I've seen countless near-misses avoided because someone recognized a hazard symbol or read the newest manual. Simple checklists cut down on unsafe stacking, careless mixing, or blocking airflow around sensitive pallets. Without these routines, even the best facility can turn risky.
Transport and Logistics Concerns
Safe storage easily turns risky during transport. Jostling, improper stacking, and changes in climate between warehouse and delivery truck all create new hazards. Using insulated containers, validated cold-chain logistics, shock-absorbent packaging, and GPS-tracked climate controls make all the difference. Supply chain teams face pressure to move fast, but speed shouldn't lead to tossing fragile, temperature-sensitive items into the wrong carrier.
Room for Improvement
As supply chains face tighter regulations and more scrutiny, technology like IoT sensors, blockchain tracking, and real-time alerts can help. These tools let everyone—producers, drivers, warehouse staff—keep an eye on crucial storage conditions. Combining proper tools with a culture of care and accountability can keep products safe, costs down, and avoid disaster from avoidable mistakes.
The Real Meaning of Purity in Modern Chemicals
Ask any experienced chemist about purity and you’ll get stories about ruined experiments, wasted money, and troubleshooting sessions that stretch into the night. It isn’t just about numbers on a spec sheet. A small impurity in N-Butyl-3-Methylpyridinium Bis(Trifluoromethylsulfonyl)Imide—often shortened to [BMPy][TFSI]—can throw a whole line of research off track or put an unexpected spin on a pilot process. Labs look for at least 99% purity. Research labs testing advanced batteries and electrolytes want no less, sometimes higher, depending on the downstream application and sensitivity of measurements. This material isn’t cheap, so nobody wants to find out the bottle from the shelf is throwing contaminants into a high-value test.
Error That Hides in the Data
[BMPy][TFSI] belongs to the ionic liquids family. These chemicals don’t mix well with water, and they don’t boil off like solvents, which is why they’re all over new research into green chemistry, energy storage, and organic synthesis. If there’s water mixed in, or if leftover solvents from synthesis stick around, the whole profile changes. I’ve seen conductivity measurements head in the wrong direction because humidity crept into a “dry” sample. Purity means less than 0.2% moisture, usually, measured by Karl Fischer titration.
Color tells its own story. Clear and pale yellow means you’re probably all right. Dark or cloudy material signals that someone got sloppy: maybe the reaction stalled, maybe starting materials stuck around, or maybe the bottle spent too long open to air. HPLC spectra should come back clean—with one dominant peak. Small bumps or doublets can signal by-products or incomplete separations. Quality control teams often use NMR and mass spectrometry to check for leftover pyridine, butyl chloride, or even rogue TFSI traces. If the report just says “conforms,” it usually means an expert signed off on those test results and nothing looks weird.
Specification That Matters in the Real World
The industry sets ranges for density, melting point, and conductivity based on loads of repeat measurements. [BMPy][TFSI] density sits around 1.4 g/mL at room temperature. Melting point falls below -20°C, which means the liquid state holds in most lab environments. Viscosity numbers, anywhere from 40-70 cP at 25°C, make life easy or hard, depending how you plan to pipette or mix it. Electrochemical window runs wide—over 4.5 volts—which helps research into supercapacitors or lithium-ion batteries.
Heavy metals rarely pop up if you’re buying from a supplier with modern equipment, though trace copper or iron sometimes finds its way in during the handling or synth of starting materials. Any spec sheet worth keeping shows heavy metal content below 2 ppm. Halogens (like chloride) also need a close look. Chloride content under 100 ppm remains the informal “good enough” cut-off, since too much leads to unwanted corrosion or catalysis issues in battery and cell applications.
Solving the Problem of Quality Control
I’ve seen plenty of small labs try to shortcut with generic ionic liquids—only to lose weeks of work chasing unexplained results. Buying from a reputable supplier helps. Always ask for batch-specific CoAs with full chromatograms and moisture data, not just “meets spec” lines. Labs in hot, humid places run dehumidifiers in storage rooms and store sample vials under argon or nitrogen to hold purity steady. Getting the pure stuff isn’t just about research pride; it’s about honest results and efficient projects that hit deadlines. Every shortcut along the way becomes a wild variable some unlucky scientist will have to fix down the road.
The Real Stakes Behind Chemical Safety Data
I’ve seen too many people brush off safety information on chemical products, treating warnings as background noise. Folks crack open a bottle, maybe give the label a half-glance, and figure someone, somewhere, must’ve checked that it's safe. Trust comes easy, even with substances we barely know. But that only works until something goes sideways.
Public health can unravel quickly when safety data gets neglected. Look at the headaches over PFAS, dubbed “forever chemicals,” now turning up in water across the globe. Decades ago, these compounds seemed harmless—at least based on the data that was available, or sometimes conveniently hidden. Only after years of manufacturing, widespread use, and actual harm did anyone dig deeper and find the real toxicity profile. People started asking tougher questions about whether producers had a duty to share what they knew, and didn’t know, with the public.
Safety and toxicity information isn’t just about meeting regulations. It means weighing real consequences for humans and the environment. The stakes get real in settings like research labs and factories, where exposure can lead to breathing trouble, cancer risks, or worse. The Material Safety Data Sheet (MSDS) might sound like paperwork, but for the folks wearing lab coats or cleaning up leaks, that sheet is the line between a safe day at work and ending up in the emergency room.
Why Gaps in Data Spell Trouble
Big gaps in safety info mean big problems down the road. In the pharmaceutical world, every vial and tablet that leaves a factory passes through layers of scrutiny—animal studies, human trials, toxicology reports. If anything gets skipped, people pay the price. In contrast, plenty of industrial solvents and additives never get that level of investigation. Some chemicals slip out to market carrying only barebones data, or worse, “no data available” scrawled in place of real answers.
Just last year, I spoke with a plastics engineer who had to replace a plasticizer because new studies found it could disrupt hormones in kids. Now, instead of just relying on what the manufacturer claims, more product developers demand independent testing and full transparency. The push for open data isn’t just ethical—it’s practical. Trusted information supports better choices for both industry and consumers.
Building Trust and Accountability
People want to feel safe—at work, at home, and in their communities. Accurate toxicity and safety data build that foundation. For trust to last, information needs to come from credible sources. Peer-reviewed studies, regulatory agency reports, and industry certifications all help, but that only works if companies actually disclose everything people should know. Relying on the “right to know” principle, regulators need to keep pressing for public access to up-to-date information, not buried in technical jargon but written so anyone can understand the risks.
Solving these challenges takes teamwork. Scientists, manufacturers, regulators, and end-users all play a part. Investing in transparent data reporting, routine safety reviews, and consumer education goes beyond compliance—it's about keeping real people out of harm’s way. The more accessible and accurate safety data gets, the smaller the chance someone gets caught off guard by a risk they didn’t see coming.