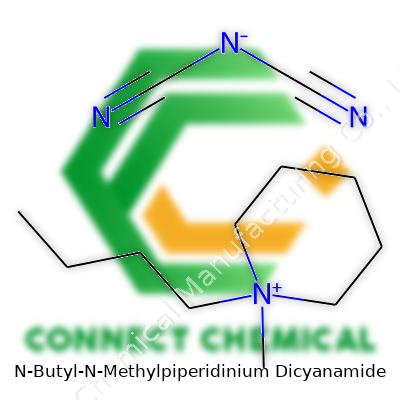
N-Butyl-N-Methylpiperidinium Dicyanamide: A Practical Look at its Place in Science and Industry
Historical Development
Scientists have searched far and wide for new ionic liquids that could handle tough industrial requirements. N-Butyl-N-Methylpiperidinium Dicyanamide entered the scene over the last few decades, marking a serious shift from traditional, hazardous organic solvents. People started looking closer at piperidinium-based salts when they realized these compounds, featuring tailored organic cations and unique anions like dicyanamide, often offered thermal stability, low volatility, and convenient tunability. As restrictions tightened on volatile and flammable solvents, this compound gained attention in research labs and on shop floors. Chemists in the early 2000s began using N-Butyl-N-Methylpiperidinium Dicyanamide for its reliable handling of high temperatures and unique solvation abilities. Since then, its reputation as a safe yet effective ionic liquid has only grown, supported by continuous work in green chemistry and electrochemical research.
Product Overview
N-Butyl-N-Methylpiperidinium Dicyanamide comes as a liquid at room temperature, clear or slightly yellowish, and holds up under a variety of storage conditions. Its unique composition—constructed around a piperidinium ring substituted with butyl and methyl groups, paired with the dicyanamide anion—brings special characteristics sought out in many fields. Usually packaged in moisture-proof containers, manufacturers make sure buyers understand it isn’t just another chemical on the shelf; its ionic nature means it conducts well even without water present. That makes it attractive for cutting-edge energy applications, specialized separations, and research into non-traditional chemical synthesis.
Physical & Chemical Properties
N-Butyl-N-Methylpiperidinium Dicyanamide holds a moderately high boiling point and melting point below room temperature, staying fluid even at low temperatures. The ionic character produces negligible vapor pressure, which reduces workers’ risk of inhaling dangerous fumes in factories and labs. Not only does the compound resist chemical breakdown, but its chemical structure limits flammability and corrosion. Its solubility varies, mixing with other polar organic liquids, but it resists dissolving in non-polar solvents. In my own work handling ionic liquids, these simple-sounding attributes save time during clean-up, minimize waste, and lessen the risk of accidental exposure. Knowing staff won’t need to suit up in full hazmat gear goes a long way in encouraging practical, repeatable research.
Technical Specifications & Labeling
Producers of N-Butyl-N-Methylpiperidinium Dicyanamide include detailed data in safety data sheets. This covers the compound’s molecular weight, chemical formula, and specific gravity. Purity often exceeds 98%. Modern supply chains label each batch with manufacturing and expiry dates, driven by tighter workplace safety standards. Labels spell out hazard warnings, emergency measures, and instructions for safe handling. Especially now, as regulatory authorities keep a closer eye on specialty chemicals, those purchasing or distributing this compound expect detailed compliance papers, batch traceability, and certification of analysis.
Preparation Method
N-Butyl-N-Methylpiperidinium Dicyanamide comes from a straightforward synthesis between N-Butyl-N-Methylpiperidine and dicyanamide source, often using a metathesis (ion exchange) reaction. Chemists combine the piperidinium salt with a sodium or potassium dicyanamide under controlled temperature in an anhydrous solvent. By removing the by-products (usually sodium chloride or potassium chloride), technicians purify the final product through washing, filtration, and evaporation steps. This approach offers a relatively mild process, reducing the risk of environmental contamination or hazardous by-products. Running this kind of synthesis at scale requires strict process controls, as small deviations in water content or reactant ratios can cause color changes, impurity formation, or yield drops.
Chemical Reactions & Modifications
One reason N-Butyl-N-Methylpiperidinium Dicyanamide attracts attention comes from its flexibility in chemical reactions. The dicyanamide anion allows for nucleophilic reactions, and the cation withstands strong acid or base conditions. Chemists tweak properties by substituting the butyl or methyl groups, adjusting for solubility, conductivity, or thermal stability. Some labs use this compound for catalysis, polymer stabilization, or electrochemical cells, because it demonstrates minimal reactivity with air and moisture. Having worked with other ionic liquids that degrade easily, I’ve seen how the robustness of this compound cuts down on unwanted side reactions.
Synonyms & Product Names
Suppliers sell N-Butyl-N-Methylpiperidinium Dicyanamide under several names, including variants such as BMPip DCA, 1-butyl-1-methylpiperidinium dicyanamide, or [BMpip][DCA]. Ordering this chemical often requires cross-referencing synonyms or catalog numbers to avoid mistakes, as minor naming differences sometimes conceal big formulation changes. Workers must double-check documentation and certificates to ensure they’re working with the intended variant.
Safety & Operational Standards
Handling N-Butyl-N-Methylpiperidinium Dicyanamide doesn’t call for extraordinary safety measures, but routine vigilance pays off. Skin and eye protection keep accidental splashes from causing irritation, and well-ventilated workspaces help manage small vapors from associated chemicals. Teams store the compound away from strong acids, bases, and oxidizers to prevent uncontrolled reactions. My experience points to benefit in periodic spill drills and careful decanting, particularly when working at larger volumes. Clearly marked containers prevent cross-contamination with more hazardous chemicals. Some organizations install local exhaust ventilation to add redundancy, but the compound’s low volatility already decreases airborne exposure risk compared to chlorinated solvents or conventional volatile organics.
Application Area
N-Butyl-N-Methylpiperidinium Dicyanamide’s real strengths show up in electrochemistry, battery development, and green organic synthesis. Research facilities explore its conductivity in high-performance capacitors and batteries. It dissolves a range of metals and organic molecules, fueling further exploration as a solvent or reactant in organic synthesis. Environmental labs examine its potential in CO₂ capture due to its strong affinity for polar molecules. My background in process research led to testing this compound as an alternative to volatile organic solvents. Results pointed to faster reaction times and reduced environmental emissions. Some companies are attempting to use it in pharmaceutical crystallization, seeking improved purity and sustainability. Universities remain committed to testing its recyclability and environmental fate, which will speak volumes about its widespread adoption in greener processes.
Research & Development
Every year, researchers publish new data on N-Butyl-N-Methylpiperidinium Dicyanamide, driven by the hunt for efficient, safe, and ecologically responsible chemical processes. Labs keep looking for new partners to blend this ionic liquid into composite electrolytes, designer catalysts, and solvent systems for renewable fuels. Partnerships between industry and academia mature each year, moving results from the bench to small production lines. Conferences brim with case studies on durability, longevity, and environmental compatibility. The compound now attracts attention from start-ups focused on emerging battery chemistries, resource recovery, and chemical recycling. Some hurdles remain; cost and regulatory uncertainty still slow down widespread adoption. Yet history shows tenacious researchers keep finding ways to solve these problems, especially as public interest in cleaner technology rises across the globe.
Toxicity Research
Early studies on N-Butyl-N-Methylpiperidinium Dicyanamide signal low acute toxicity in mammals compared to legacy solvents, but no scientist claims it’s entirely harmless. Inhalation or direct injection studies highlight mild irritation, while longer-term environmental impacts are still under review. Persistence, bioaccumulation, and long-term breakdown in soil or water are topics under scrutiny by regulatory bodies and toxicologists. Some reports suggest the compound breaks down more quickly than traditional ionic liquids; independent studies verify these findings with targeted aquatic toxicity panels. My advice sticks with the tried-and-true: handle with respect, use in closed systems, and never dump down the drain. Future areas of scrutiny might expand to endocrine disruption or reproductive risk—issues that often slip by in early product evaluations.
Future Prospects
N-Butyl-N-Methylpiperidinium Dicyanamide’s importance keeps rising as pressure mounts for sustainable chemical solutions. If recycling and disposal methods continue to mature, the compound’s popularity in green synthesis and energy storage will only grow. Upstart research in CO₂ capture and recovery, next-generation batteries, and safe process scale-up points to even broader markets ahead. Barriers like price, inconsistent regulations, and incomplete data fade in the face of bigger scientific and economic pushes. Direct experience running trials or scaling up production lines makes clear: real-world performance, clear labeling, and reliable sourcing often decide which specialty chemicals stick around. Unless unexpected red flags surface about long-term toxicity or environmental impact, this ionic liquid could anchor countless chemical technologies in years to come.
The Quiet Powerhouse Behind Batteries
N-Butyl-N-Methylpiperidinium Dicyanamide pops up more often in the guts of new battery technology than most would think. What brings it here is its knack for forming room temperature ionic liquids. The chemicals behind tomorrow’s batteries need to handle temperature swings and provide stability you just don’t get from many older salt or solvent systems. This substance touches on those needs in a real way. Lithium-ion batteries, for example, face burning questions on fire safety as more homes and vehicles charge up. Researchers have shown that swapping typical solvents for dicyanamide-based ionic liquids drives battery safety forward by ditching flammable components, keeping performance high, and life cycles long. It’s not just about making batteries last, but keeping homes and workplaces safer, too.
Greener Chemistry: Solvents Without the Risk
One thing I have always appreciated about chemistry is the push for safer, more sustainable solutions. Dicyanamide-based ionic liquids sit at this crossroads. They can dissolve a staggering range of organic and inorganic compounds, which brings flexibility to chemists chasing new reactions or greener syntheses. I’ve read plenty of studies showing these liquids can swap in for harsher, more toxic solvents in pharmaceutical or material production, cutting back waste and lessening the strain on air and water. Factories using fewer volatile organic compounds help keep communities healthier and operations on the right side of environmental regulation.
Fuel Cells and the Search for Clean Energy
Clean energy doesn’t just need a new kind of fuel. It needs the right fluids and electrolytes. Dicyanamide salts, especially paired with piperidinium, are carving out a spot inside fuel cells. The reason is pretty straightforward—these compounds deliver stable conductivity, stand up to heat, and don’t let their structure slip when loaded into a cell. Research out of Europe and Japan highlights how swapping conventional protic acids for dicyanamide ionic liquids raises efficiency and slashes degradation. This makes sense to me, since fuel cell designers aren’t only aiming for performance, but for tech that actually holds up after years of cycling between charge and discharge. Lower maintenance bills and better uptime attract real-world applications for buses, trains, and even backup power.
Putting the Pieces Together: Issues and Ways Forward
The industry interest isn’t only about performance. Sourcing, toxicity, and cost play a huge role in how far these new compounds spread. Making dicyanamide-based liquids at industrial scale means tackling rigorous safety checks. That’s warranted, since new substances—especially those bound for consumer goods—shouldn’t bring new risks to people or the planet. Some studies point out the need to monitor breakdown products and their impact on soil and water. Regulatory scientists deserve credit here—they keep potential incidents in check and push for better data from all producers. For this kind of technology, more open testing and collaboration will clear up these safety questions.
A broader solution draws from what’s worked in the past. When inventors, regulators, and end-users get in a room—sharing real-life data and feedback—it steers new compounds like N-Butyl-N-Methylpiperidinium Dicyanamide toward responsible use. This creates space for both safer batteries and cleaner factories, helping more people trust what’s powering their future.
Understanding Chemical Stability
Stability sets the ground rules for working with any compound. If a chemical breaks down under normal conditions, it can create hazards, lose potency, or even transform into something nobody expects. A common issue in many labs involves reagents that slowly degrade on the shelf, even with the bottle sealed tight. I've seen this more than once — a fine white powder turning yellow at the edges, or a liquid growing cloudy, just weeks after opening.
Temperature, humidity, and light play major roles in keeping a material from decomposing. Some compounds tolerate room temperature just fine; others need refrigeration or even deep freezing. You can't always rely on intuition here. Once, a simple oversight — leaving a light-sensitive chemical on a sunlit bench — led to an entire batch losing its effectiveness. Ultraviolet rays triggered a reaction, and what looked like normal powder stopped working as intended.
Storage Conditions Define Safety and Quality
The consequences of poor storage often go unnoticed until trouble knocks. Volatile solvents kept in open containers will evaporate or absorb moisture, skewing concentrations and results. Corrosive materials start to degrade their surroundings, rusting lids and weakening plastic containers. In cases involving oxidizers or reactive agents, poor storage can push things from inconvenient to downright dangerous. More than one warehouse has learned this lesson the hard way, dealing with fumes, sticky floors, or worse.
Labeling matters — not just what’s in the bottle, but when it was made, and how it should be handled. An unlabeled flask doesn’t just risk mistakes; it undermines trust in the whole workflow. Take it from years at the bench: urgency or shortcuts in labeling end up costing more time and wasted resources.
Practical Solutions for Reliable Storage
Making stability part of your routine protects both your results and everyone nearby. Closed, chemical-resistant containers cut down on moisture and air exposure. Silica gel packs or desiccators can help keep powders dry, especially if you live or work in humid environments. Light-sensitive compounds need storage in amber bottles or, better yet, in dark cabinets. In our lab, routine audits of the fridge or chemical closet uncovered forgotten reagents before they turned into problems. Simple practices like this stopped expired materials from getting into new experiments.
Many chemicals arrive with data sheets outlining temperature and humidity limits. Taking those recommendations seriously isn’t an academic exercise. Ignoring them risks wasted funds, unpredictable performance, and sometimes legal headaches. Refrigerators sound like overkill for simple compounds, until the results tell you otherwise. More than once, shifting a reagent from the bench to cold storage kept it potent for months longer.
Busy teams can benefit from tracking software or shared documents. By keeping an updated log of what’s stored, expiration dates, and opening dates, the whole group pulls in the same direction. I’ve watched labs turn around safety and reliability simply by organizing shelves and keeping a whiteboard updated.
The Real-World Impact
Getting storage and stability right isn’t just about ticking regulatory boxes. Good practices keep people safe, protect investment, and lead to trustworthy data. They prevent disasters nobody wants to clean up and ensure tomorrow’s work isn’t sabotaged before it starts. If there’s doubt, ask a colleague, check the data sheet, or lean on experience — because cutting corners here never pays off in the long run.
A Hard Look at Chemical Hazards
Anyone who’s ever opened a chemical catalog probably remembers seeing names like N-Butyl-N-Methylpiperidinium Dicyanamide. These ionic liquids pop up in research for batteries, solvents, and new tech that’s hungry for efficient electrolytes. With a name so long, it’s tempting to trust the lab supplier’s description and dive in headfirst. But the question really comes down to: is it safe, or are we tossing another sleeper hazard onto the workbench?
Chemistry’s Unfamiliar Territory
Now, in grad school I spent hours at a fume hood, wrestling with chemicals that had MSDS sheets thicker than my lunch. Ionic liquids like N-Butyl-N-Methylpiperidinium Dicyanamide have a reputation for being non-volatile, which some folks associate with lower risk. Less stink in the air seems reassuring. But digging through the available studies, it doesn’t take long before red flags start popping up.
Dicyanamide compounds, as a group, carry meaningful safety baggage. The breakdown products, especially under heat or acidic conditions, can include hydrogen cyanide. No one wants to learn the hard way how toxic hydrogen cyanide gas can be. Simple splashes that get on the skin can also lead to absorption, and it doesn’t take an expert to spot trouble there. My own gloves have turned sticky from a spill, and even a short delay in clean-up can make a difference. Sure, no dramatic fumes, but hidden risks often start like that.
Manufacturer Information Isn’t Always Enough
I’ve noticed MSDS sheets for these ionic liquids sometimes rely on data from similar chemicals, not the exact one being handled. That leads to uncertainty. Chronic toxicity studies don’t cover all corners—long-term risks to organs, developmental effects, even mutagenicity sometimes remain unaddressed. People trust suppliers not to oversell the safety profile, but in academic and industrial settings, new solvents tend to fly under the radar right up until someone gets sick.
A 2022 review article highlighted that dicyanamide-based ionic liquids can mess with aquatic life at low concentrations, affecting fish embryo development and algae growth. If it leaks down the drain, it doesn’t vanish—it lingers, potentially making trouble in the environment. Occupational exposure limits haven’t been cemented by OSHA or EU regulators. So, without official benchmarks, researchers depend on best practices, sometimes improvising safety decisions. Every lab worker deserves better than educated guessing.
Getting Safer: Simple Shifts with Big Impact
A big step forward means treating unfamiliar ionic liquids like this one with full-blown respect. Fume hoods aren’t just window dressing; they protect against unexpected off-gassing and aerosolization you sometimes miss on the first pour or during disposal. I always double glove with nitrile—cotton liners underneath help avoid skin absorption. Proper labeling and secure containers beat the urge to reuse glassware "just this once." Above all, question “inert” claims—hidden breakdown products and byproducts challenge a lot of assumptions.
Labs can step up waste management by using specialized disposal for cyanide-containing waste. Simple tracking of exposures—who used it, when, how much—lets everyone spot patterns if symptoms or hazards emerge later. Peer-reviewed data collection goes further than manufacturer blurbs, pressing for independent hazard analysis. Until broader toxicological data roll in, it makes sense to apply the highest safety standards we’d use for more famous hazards.
Responsible Science Demands More Than Guesswork
Cutting-edge chemistry brings real opportunity, and new solvents power next-gen devices, but every progress leap asks for honest risk-taking—not just in research, but in how we protect the people making those discoveries. As scientists and engineers, asking the tougher questions about safety and pushing for transparency puts everyone in a better spot, in the lab and beyond.
The Real Meaning of Purity
Purity gets tossed around a lot in labs and industry talk, but outside the textbook, it tells a clear story about how much of a product is actually the real deal. For chemicals that end up in pharmaceuticals, food, or high-stakes manufacturing, purity doesn’t just mean high quality on paper. It brings peace of mind for everyone along the supply chain. Usually, when labs report purity, they mean the percentage of the main ingredient compared to everything else mixed in. For high-grade products—think active ingredients or reagents—purity often runs over 99%. In my own work, anything under 98% raises eyebrows immediately, since it can throw off results or even ruin a batch.
Every report or label has to back up those purity numbers. Labs usually run thin-layer chromatography, high-performance liquid chromatography, or other tests. Having a certificate of analysis that matches those numbers helps buyers trust what they’re getting. Even a 1% impurity in a crucial synthesis step will sometimes derail days or weeks of effort or, in production, lead to batches that fail quality testing. This isn’t just theory—companies have lost customers over small slips.
What to Look for in Appearance
Most folks think all chemical powders look about the same, but chemists know appearances can signal real trouble or show when something’s gone right. For a lot of pure products, you want to see a consistent color and no weird clumping—maybe a white crystalline powder or a clear, colorless liquid. The product should match the description in standards documents. If something turns up with discoloration, unexpected clumps, or a strange smell, it’s often a warning that the batch contains impurities or has absorbed water.
I once opened a container expecting a solid white powder and found a slightly yellowed mass stuck together. Turns out, the product had absorbed moisture during shipping. That small change pointed to possible breakdown of the material and could have made any experiments fail. Companies with quality controls check appearance just as strictly as chemical composition, knowing that customers rely on these visual checks to flag problems even before running full analyses.
Real-World Impact of Overlooking Purity and Appearance
Impurities in chemical products can create headaches long before they make headlines. A contaminated solvent in cleaning processes, for example, can leave unwanted residues on machinery parts. In pharmaceuticals, FDA recalls often start with a single off-color batch, even before tests confirm the presence of impurities. End users need both a clear certificate and straightforward inspection guidelines to catch problems early.
Paying attention to external clues—the way a powder flows, the color, the label accuracy—means less waste, fewer failed runs, and stronger trust between buyer and supplier. Training staff to judge appearance isn’t just nitpicky; it can help avoid costly mistakes and even protect health. Too often, labs chasing tight budgets will overlook visual checks or skip confirming certificates and end up paying more down the line.
Moving Toward Solutions That Stick
Quality in raw materials rarely just happens. Suppliers who share clear certificates, provide reference images, and keep tight controls make a difference. On the lab side, building routines—verifying every batch against known standards, periodically retraining staff, and rejecting containers that seem suspect—prevents trouble. Even something as simple as keeping storage conditions steady and documenting how materials looked on arrival can make future problems easier to trace. No one fix will keep all impurities at bay, but stacking these habits puts control back in the hands of the people who need it most.
Accessing Specialty Chemicals
Researchers and tech developers searching for N-Butyl-N-Methylpiperidinium Dicyanamide often start with scientific supply chains before turning to specialty dealers. Sigma-Aldrich, Alfa Aesar, and TCI America remain among the suppliers regularly referenced in industry discussions. Each company maintains strong reputations for product traceability and batch consistency, key issues for folks producing reliable research data or pushing toward scale-up manufacturing.
Packaging Sizes and Practical Considerations
You won’t spot this compound sitting on the shelves of your neighborhood hardware store. Chemical distributors usually offer it in quantities tailored for R&D or pilot projects. Most outlets sell it in sealed glass or high-density polyethylene bottles, starting at about 5 grams and scaling up to 100-gram or 500-gram containers. The jump to kilogram-sized purchases usually requires more paperwork and vetting—suppliers want to make sure their product goes to qualified buyers for legitimate uses.
For a grad student or bench chemist in a small organization, the five-gram and ten-gram bottles let you experiment without committing to expensive inventory. Those working in battery labs or ionic liquid research often need higher purity in these small lots, and top suppliers back up every order with detailed documentation. Larger labs, or companies moving out of the sandbox stage, benefit from 500-gram sizes or custom packaging negotiated through sales representatives. Every container needs to be compatible with the chemical’s stability profile, so suppliers invest in packaging designed to reduce risks of leaks or degradation.
Quality, Regulation, and Buyer Responsibility
N-Butyl-N-Methylpiperidinium Dicyanamide is used in advanced battery research and other applications involving ionic liquids. With these kinds of chemicals, quality matters. Impurities can wreck experiments or throw off performance data. Top suppliers earn trust through batch testing and strict supply chain controls. Each bottle usually arrives with certificates of analysis detailing purity, moisture content, and any unusual observations during production.
Regulations around the shipping and purchase of this compound vary by country. In my experience, import permits, end-use declarations, and destination-specific paperwork can slow things down. Buyers in North America and Europe will find that most distributors enforce “know your customer” checks. The best way to navigate this is to keep communications open, explain your research or application needs, and be ready to provide institutional credentials.
Finding a Solution to Supply and Access Gaps
Global supply chain disruptions taught many researchers that relying on a single supplier isn't wise. Establish agreements with two or more distributors if possible. In 2022, a friend working on sodium-ion batteries experienced a three-month delay after her usual supplier ran out. She got back on track after reaching out to a competitor who offered a different packaging size than she usually bought. Adaptability helped her keep the project moving, even if it meant adjusting batch sizes and storage space.
Always ask about freshness, storage temperatures, and lead times. Demand for niche chemicals can surprise suppliers when a new field of research heats up. Secure contracts or pre-orders help labs avoid getting caught without the materials they rely on, and frequent communication gives both sides a chance to spot issues before they turn into project delays.
Looking Ahead
As industries keep exploring next-generation energy and solvent solutions, more distributors will carry N-Butyl-N-Methylpiperidinium Dicyanamide in wider packaging varieties. Buyers who value transparency, quality verification, and flexible volume options tend to navigate the market with fewer hiccups and greater confidence. Networking with peers, keeping an eye on supplier quality, and asking the right questions will ensure smooth progress in any cutting-edge project.