N-Butyl-N-Methylpyrrolidinium Acetate: An In-Depth Commentary
Historical Development
You don’t need to dig too deep in chemical literature to see how the world of ionic liquids started changing as early as the 1990s. N-Butyl-N-Methylpyrrolidinium Acetate came onto the scene once researchers began searching for safer, more sustainable chemical solvents with lower volatility. Early work in ionic liquids mostly revolved around imidazolium-based compounds, but interest grew fast in pyrrolidinium variants for their higher thermal stability and less reactive cationic cores. Over several decades, labs in Europe, North America, and Asia found strong reasons to look past traditional salts and organic solvents, especially for applications needing wide thermal windows and improved safety profiles. The route to scale-up ran through years of trial-and-error synthesis and close attention to purity. Each batch of N-Butyl-N-Methylpyrrolidinium Acetate added to the collective knowledge, leading to robust preparation techniques and more use cases.
Product Overview
N-Butyl-N-Methylpyrrolidinium Acetate enters the market as a clear, somewhat viscous ionic liquid. Chemically, it contains a pyrrolidinium core with butyl and methyl substitutions on the nitrogen, and an acetate anion settles the whole thing into an ionic liquid form at room temperature. No significant odor, good shelf stability, and predictable behavior make it a favorite in solvent systems and as a building block for advanced materials. You find product vials with purity specifications ranging upward of 98%. It usually comes packaged in amber glass bottles because exposure to light and air gradually degrades the acetate component. Large-scale industrial quantities get shipped in polymer-lined drums. Batch records and lot numbers go along with every order so traceability stays intact from lab bench to pilot plant.
Physical & Chemical Properties
At room temperature, N-Butyl-N-Methylpyrrolidinium Acetate sits as a colorless to pale yellow liquid. Its density averages about 1.07 g/cm³ at 25°C, showing very little volatility under ambient conditions, which reduces exposure in handling. Viscosity measures can vary by supplier batch, with range estimates falling between 80-150 centipoise at room temperature. Water solubility runs high, but miscibility with apolar solvents drops off, thanks to both the hydrophilic acetate anion and polar pyrrolidinium core. Its decomposition temperature clocks in at over 200°C, and it resists most spontaneous exothermic breakdowns. As an ionic liquid, its conductivity depends on temperature and water content but supporting electrochemical experiments shows stable performance in custom battery electrolytes and as a co-solvent in catalysis. Chemical inertness allows it to persist in complex reaction mixtures while not altering the progress of acid- or base-catalyzed processes unless specified.
Technical Specifications & Labeling
Spec sheets for N-Butyl-N-Methylpyrrolidinium Acetate usually detail minimum purity, moisture level, and color index. Customers look closely at heavy metal content and residual starting material—n-methylpyrrolidine or butyl bromide, for instance. Certificates of Analysis state melting point (often -13°C or lower), along with key infrared and NMR spectral data to confirm structure. Chemical labeling needs to reflect hazard classification—most jurisdictions require GHS pictograms for skin and eye irritation unless data show otherwise. Larger shipments get UN identification and handling instructions for chemical compatibility during transit. Manufacturers highlight the need for cold storage only if product testing reveals rapid hydrolysis or acetate loss at higher temperatures. Batch traceability and expiration dates form an important part of quality assurance, especially for users following GLP or GMP regimes.
Preparation Method
Laboratory synthesis of N-Butyl-N-Methylpyrrolidinium Acetate follows a straightforward path, often taught in advanced organics courses. Start with N-butyl-N-methylpyrrolidinium bromide—this intermediate forms from alkylating N-methylpyrrolidine with butyl bromide in a polar aprotic solvent. The next step uses metathesis: React the bromide salt with sodium acetate in water or methanol. Stir, filter off sodium bromide, and then remove solvent under vacuum to leave the crude ionic liquid. Final purification uses charcoal treatment and vacuum drying until color and IR shifts signal full product cleanup. Industrial setups run these steps in larger jacketed reactors under nitrogen, always watching for moisture contamination—the acetate salt can absorb water from the air and impact both yield and downstream performance.
Chemical Reactions & Modifications
N-Butyl-N-Methylpyrrolidinium Acetate works well as an inert solvent for a spectrum of organic transformations. You see it act as a reaction medium for palladium-catalyzed couplings and as a stable electrolyte base in ionic conductivity studies. The pyrrolidinium cation resists nucleophilic attack way better than imidazolium cousins, so it stands up under harsh basic or acidic conditions. That said, the acetate anion can undergo mild nucleophilic substitution and serve as a hydrogen bond donor in supramolecular chemistry. Researchers sometimes replace the acetate with other carboxylates using simple salt metathesis to tune solubility for new applications, especially in green electrochemistry. In custom polymer synthesis, this ionic liquid enters as a functionalized monomer or plasticizer, allowing unique modifications of chain flexibility and ion transport.
Synonyms & Product Names
N-Butyl-N-Methylpyrrolidinium Acetate shows up on catalogs and publications under a handful of synonyms. Common ones include BMPyr Acetate, [BMPyrr][OAc], and 1-butyl-1-methylpyrrolidinium acetate. CAS number for accurate identification is 799276-30-9. Some distributors list it as an ionic liquid or green solvent, but always scan the technical data to ensure correct isomer and counterion.
Safety & Operational Standards
My own experience working with N-Butyl-N-Methylpyrrolidinium Acetate taught me the importance of setting up good lab discipline even for lower-risk chemicals. The main hazard lies with eye and skin contact, owing to the acetate’s mild irritancy. Wear nitrile gloves and splash-proof goggles every time you transfer or weigh. Workstations benefit from laminated safety cards listing first-aid steps and chemical compatibility, especially since mixing this liquid with strong acids or oxidizers triggers unwanted side reactions. Spill cleanup is straightforward—just absorb with a neutral pad and rinse with water—but any exposure should prompt immediate washing under running water for at least 15 minutes. Most suppliers recommend storing under dry nitrogen and away from direct sunlight, using secondary containment. These cautious habits protect both user and product integrity, especially in high-value experiments or scale-up runs.
Application Area
N-Butyl-N-Methylpyrrolidinium Acetate fills roles across fields like electrochemistry, catalysis, analytical chemistry, and advanced materials. Research teams run it as a lithium-ion battery electrolyte, thanks to high ionic conductivity and low volatility. Solvent properties support metal-catalyzed transformations, dissolving both polar and semi-polar reagents. In carbon capture projects, the combination of acetate anion and pyrrolidinium cation stabilizes CO₂ loaded phases while keeping regeneration energy costs down. I’ve seen labs integrate this ionic liquid into cellulose processing, where it breaks down tough biomass structures for renewable fuel research. You also find publications describing use in lubricants, paints, and functional coatings. In microfluidic chip design, N-Butyl-N-Methylpyrrolidinium Acetate acts as a non-volatile, precisely-tuned phase for sensor calibration or embedded microreactors.
Research & Development
Research on N-Butyl-N-Methylpyrrolidinium Acetate keeps expanding. Universities and private labs explore electrolyte performance in solid-state batteries, where this ionic liquid’s temperature window beats traditional options. I know small startups working with it as a CO₂ absorbent; the reversibility of its binding offers big energy savings. Machine learning models crunch its thermodynamic data for design of new solvents. Funding agencies demand comparative life cycle analysis, so data availability on product energy use and end-of-life impact gets factored into every grant proposal. Open-access databases now track published reaction conditions and yields for processes using this ionic liquid. These resources move the field from isolated discoveries to standardized protocols, improving reproducibility and drawing new collaborators from engineering and applied science communities.
Toxicity Research
Available toxicology reports show low acute toxicity for N-Butyl-N-Methylpyrrolidinium Acetate, especially compared to volatile organic solvents it replaces. Chronic exposure data is limited, but most findings point to skin and eye irritation as the primary risks, with only trace evidence of mutagenicity or cytotoxicity in cell models at typical concentrations. Environmental persistence of ionic liquids poses a challenge: acetate breakdown in soil moves faster than more complex anions, but the pyrrolidinium core resists microbial degradation. Some work suggests measurable aquatic toxicity at higher concentrations, prompting regulatory agencies to push for improved waste treatment guidance. In lab and plant settings, waste collection and proper disposal avoid local discharge, so responsible waste policy should remain a top priority. Chemical monitoring programs track down-the-drain loss to ensure N-Butyl-N-Methylpyrrolidinium Acetate stays within permissible exposure limits—a field where future research should aim to fill data gaps.
Future Prospects
Looking ahead, I expect N-Butyl-N-Methylpyrrolidinium Acetate to keep rising in green chemistry toolkits. Battery manufacturers show heavy interest, searching for ionic liquids that balance safety, performance, and recycling. Efforts to scale up production could drive down per-kilo costs, making it practical in large-scale separations and plant-based biomass processing. Researchers across Europe and Asia look into hybrid ionic liquid systems, blending this acetate with others to hit custom properties for heat pumps and biopolymer extractions. Advances in toxicity screening tech will address concerns on environmental and biological impact, letting regulators close current loopholes. Cross-industry collaboration—from solvent makers to energy storage projects—holds the key to new use cases. A reliable supply chain of high-quality N-Butyl-N-Methylpyrrolidinium Acetate, tied to ongoing safety reviews and cleaner disposal protocols, could shift industrial chemistry toward safer, greener practices within the decade.
An Ionic Liquid With Real-World Impact
The world has plenty of hard-to-pronounce chemicals, and N-Butyl-N-methylpyrrolidinium acetate counts among them. Despite its name, it finds real use—you’ll spot it in advanced labs and across new industries. Over the last decade, scientists have looked to ionic liquids like this one as replacements for old-school solvents and additives. Traditional solvents, like acetone and toluene, cause headaches—literally and figuratively. They create waste and health risks. By comparison, N-butyl-N-methylpyrrolidinium acetate shows lower toxicity and better stability at high temperatures.
Biomass Processing: Making Use of Tough Materials
Think about agricultural leftovers: corn stalks, wood chips, rice husks. These tough materials, known as lignocellulosic biomass, just sit there for the most part. Breaking these down usually requires harsh chemicals. N-Butyl-N-methylpyrrolidinium acetate offers an option that works at lower temperatures and leaves behind fewer byproducts. Researchers found that it dissolves cellulose efficiently. Factories making biofuels or biodegradable plastics could benefit. Using an ionic liquid with these properties might cut pollution and lower running costs.
Safe and Green: Replacing Hazardous Solvents
Plenty of industries run on solvents. Paints, coatings, pharmaceuticals—all use them. Old-school solvents evaporate fast and harm workers. N-Butyl-N-methylpyrrolidinium acetate stays in liquid form over a wide range of temperatures without releasing harmful vapors. In the pharmaceutical field, it helps extract valuable compounds from plants and assists in chemical reactions. Plants that used to require costly ventilation now find relief with less vapor and fewer risks for fires.
Materials Science and Electronics
Modern electronics keep shrinking in size. Ionic liquids play a behind-the-scenes role. This particular liquid acts as an electrolyte in advanced batteries and supercapacitors. Unlike the flammable mixtures in many lithium-ion batteries, it resists combustion. That means electronics using it stand a better chance against fires—even under stress. Some companies have tested it in solar cells and special coatings that resist corrosion. In these cases, the acetate part helps transport ions smoothly, which translates to better performance.
Challenges and Opportunities For Adoption
Switching to an unfamiliar liquid takes guts. Some manufacturers voice concerns over price. N-butyl-N-methylpyrrolidinium acetate costs more than basic solvents. Yet, the benefits start stacking up for industries aiming to lower emissions or avoid harsh work conditions. The long lifespan of this chemical means fewer replacements during long manufacturing runs. Plus, efforts are underway to recycle and reuse it after purification. Forward-thinking companies research methods to lower the cost of synthesis by tweaking the production route.
Looking Toward Sustainable Manufacturing
It’s easy to overlook the fluids running through a chemical plant. Over time, though, safer and more sustainable options change how business gets done. Researchers and process engineers see ionic liquids such as N-butyl-N-methylpyrrolidinium acetate as building blocks for a safer, greener industrial landscape. If costs come down and recycling gets easier, its use will keep spreading—making high-tech industries safer and more responsible. That has real meaning for workers, communities, and anyone keeping an eye on a sustainable future.
Chemical Formula and Structure
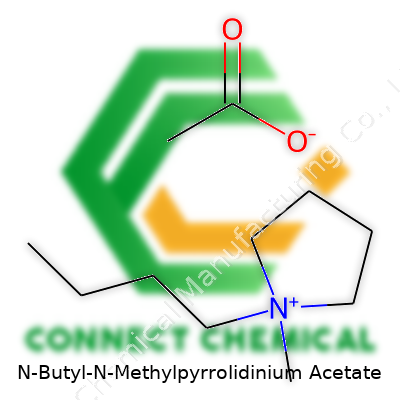
N-Butyl-N-methylpyrrolidinium acetate carries a rather hefty name, but its composition is straightforward if you look at it piece by piece. The chemical formula is C12H25NO2, which breaks down into its main components: the N-butyl-N-methylpyrrolidinium cation and the acetate anion. The cation comes from a five-membered pyrrolidine ring, with a methyl group and a butyl chain attached to the nitrogen atom. Acetate brings the familiar CH3COO- group to the table, well-known from everyday compounds like vinegar.
The structure of N-butyl-N-methylpyrrolidinium acetate reflects the approach of many modern ionic liquids: pairing a bulky, asymmetrical organic cation with a simple organic anion. The pyrrolidinium ring keeps the cation rigid but leaves flexible points where side chains can swing. The butyl tail brings oil-like character, changing how the molecule moves in a solution and interacts with other substances. Wedging a methyl group onto the nitrogen tweaks both melting point and solubility. The acetate ion, small and charged, interacts with the cation through electrostatic attraction but isn’t prone to forming crystals at room temperature. The result is a salt that tends to stay liquid well below water’s freezing point.
Why This Matters for Real-World Problems
People look to compounds like N-butyl-N-methylpyrrolidinium acetate for jobs traditional salts just can’t handle. Most folks wouldn’t think much about melting point while working in a lab, but once you see how stubborn traditional salts are about staying solid, a liquid salt at room temperature begins to look pretty useful. The unique pairing in this compound gives it a low melting point and very low volatility, unlike old-fashioned solvents or molten salts. Those advantages turn into practical solutions for chemists hunting safer, greener ways to dissolve, extract or process materials.
In my own work around cellulose research, I’ve seen firsthand how a well-designed ionic liquid changes the game. Standard solvents often fall short when trying to break down cellulose, a tough plant polymer that resists most chemicals. With N-butyl-N-methylpyrrolidinium acetate, I’ve watched stubborn fibers dissolve more readily, opening doors for bioplastics and renewable materials. Compared to imidazolium-based salts, the pyrrolidinium framework brings better stability in both acidic and basic conditions, important for repeated cycles of use in real industrial setups.
Potential and Limitations in Practice
These ionic liquids step up on the safety front as well, given their almost-negligible vapor pressure. That translates into less inhalation risk in the lab and fewer losses to the environment, especially compared to the solvents I dealt with early on in my research career. Acetate’s reputation as a relatively “benign” anion means the toxicity profile can be better — although, as always, nobody should pour this stuff down the drain without proper treatment.
Cost and availability stand as the main barriers for many researchers and industries considering a switch. The synthetic steps for the pyrrolidinium cation are more involved than making sodium acetate or simple alcohols. Scalability remains limited to specialized suppliers, making it expensive if someone needs large quantities. Purity plays a big role in performance, and minor impurities can cause big headaches in more demanding chemical reactions. For these liquids to reach broader markets beyond the lab, smarter routes to high-purity production would help a lot.
Looking at Solutions
Researchers and industry specialists keep chipping away at these problems. Direct synthesis routes and work-up steps have seen some improvement, thanks to better catalysts and continuous processes. Partnerships between universities and chemical manufacturers are starting to pop up, focused specifically on lowering costs of ionic liquids like N-butyl-N-methylpyrrolidinium acetate. Creating recycling methods also matters. Reusing the ionic liquid after extraction or reaction not only saves money but also keeps waste down, a lesson anyone working with hazardous chemicals will appreciate.
What We Know About This Chemical
N-Butyl-N-Methylpyrrolidinium Acetate comes up more often these days in labs and green chemistry. Scientists like it for dissolving cellulose and working in energy projects. It stands out as one of those “ionic liquids,” which folks see as less volatile and, at times, less flammable than the regular old solvents. Because it’s used around people doing research, safety can’t be just a side note.
How Toxic Is It?
Researchers haven’t found vast piles of public data on how this compound acts in the body, but a few things stand out. It contains an acetate anion and an organic cation. There’s no record of it spreading as a poison or anything, but that doesn’t mean it gets a free pass. Classic ionic liquids sometimes cause issues with skin, eyes, or breathing if you let them touch you or linger in the air. Some break down slowly in the environment, which means they can stick around longer than you would want.
European regulators classify ionic liquids like this as worth handling with care. Studies track effects on aquatic life and on mammals. Some relatives in this chemical family caused cell stress, even cell death, if researchers cranked up the dose in lab tests. At the same time, a few tested forms looked less harmful than old-school solvents. Bottom line—if you splash this on your hands or breathe it in by accident, your body might not be happy.
Why Handling Matters Most
I’ve worked in labs where people don’t take anything for granted. One busy afternoon, someone dropped a beaker with a similar ionic liquid. Gloves did their job, but the smell hung around—you can’t see vapors, but you quickly remember to check the safety sheet. For N-Butyl-N-Methylpyrrolidinium Acetate, the basic steps hold: goggles, gloves that actually resist solvents, and fume hoods. Spills need cleanup with care, not with bare hands or rags you toss in the trash.
This isn’t just bureaucracy. The U.S. Occupational Safety and Health Administration and the European Chemicals Agency remind people to treat chemicals with respect, especially new ones that show promise in industry. You don’t want to let curiosity lead to a trip to the hospital.
Environmental Risks and Disposal
Besides personal health, we can’t skip how these ionic liquids affect rivers, soil, or wildlife. Early research shows that some stick around if they spill, and fish or aquatic bugs might not bounce back. Used chemicals never belong down the drain. Proper collection, labelling, and follow-up with waste professionals keep it out of local water systems and back yards.
How To Stay Safe
Sticking with the basics works. Read up on the safety data sheet. Use the right gear. Store the bottle somewhere cool and dry, away from acids or bases. Don’t eat lunch at your bench. After each use, wash your hands, even if you wore gloves. If you spill it, let your safety team know so they can help.
Scientists, teachers, and techs all want safer chemicals, but newer doesn’t always mean non-toxic. Until long-term studies roll in, the best path sticks with caution, smart habits, and honest questions. That keeps work moving forward without bruising anyone’s health in the process.
The Real-World Context of Storing This Ionic Liquid
N-Butyl-N-Methylpyrrolidinium Acetate isn’t a chemical you run across at your neighborhood hardware store, but in labs and industrial operations, more people are working with ionic liquids like this one every year. Without proper storage and handling, health and safety slip out the back door, fast. The chemical's unique combination of low volatility and high thermal stability looks great on a spec sheet but doesn't mean you can toss it anywhere.
What Every User Should Know About Storage
Direct sunlight is a no-go. Strong light and fluctuating temperatures will start to break this liquid down or, at the very least, tweak its properties. A dry, cool spot—think in the range of 15 to 25 degrees Celsius—gives the best shot at longevity and reliability. Humidity can contaminate or degrade the purity, and that leads to lousy performance in sensitive processes, especially across green chemistry or catalysis projects.
Metal shelves, open stacks, or shared chemical cabinets raise red flags. In my own experience, even a splash of organic solvent or trace acid kicked off unpredictable reactions. Dedicated, closed containers made from compatible plastics or glass, tightly sealed, set the golden standard. Proper labeling—date received, supplier, hazards—becomes essential, not just for safety officers but for every staffer grabbing a bottle in a rush.
Personal Safety Matters: Handling Procedures
No one wants chemical burns or inhalation problems at work. N-Butyl-N-Methylpyrrolidinium Acetate has a reputation for low volatility, but it isn’t entirely safe on skin or in lungs. Gloves, chemical goggles, and a lab coat aren’t optional—they’re your everyday kit. After seeing one too many allergic skin reactions, I can say skipping gloves is penny-wise, pound-foolish.
Ventilation holds a critical position, even if fumes don’t seem obvious. Fume hoods aren’t an academic luxury; they really do keep tiny airborne concentrations where they belong—away from your lungs. Clean spills immediately with disposable wipes, then dispose of them as hazardous waste. The sticky, slightly oily texture of this ionic liquid means cleaning isn’t as simple as a quick paper towel swipe.
Troubleshooting and Avoiding Common Pitfalls
Improper waste management creates headaches fast. Ionic liquids complicate standard hazardous waste routines because mixing them with common solvents or water sometimes produces unpredictable byproducts. Dedicated, clearly marked waste containers for this chemical prevent confusion and accidents. Consulting your local safety data sheets can save the day; European Chemical Agency guidance and supplier recommendations both go deeper than those old binder printouts ever did.
Every year, I see teams underestimate the risk from mixing multiple chemicals in a single hood or on shared workspace benches. Cross-contamination isn’t just theoretical—tiny residues left unchecked have ruined samples and sent workers to urgent care.
Working Toward Safer Labs and Shops
Routine self-audits catch slip-ups long before accidents happen. A careless storage arrangement or forgotten cleanup, left unchecked, quickly snowballs into a major safety issue. Maintaining written protocols and regular training, tailored to each staff member’s actual job, keeps safety at the forefront even when things get hectic.
In the end, the small effort of handling N-Butyl-N-Methylpyrrolidinium Acetate correctly pays off with fewer headaches, better results, and a safer place to work for everyone involved. That’s the kind of outcome every lab should aim for.
Real-World Sourcing in 2024
N-Butyl-N-Methylpyrrolidinium Acetate catches a lot of attention in chemical research and certain industries, especially among folks working on green chemistry. Here’s the thing: not every online store or chemical catalog lists this compound. Most general consumers won’t find it through regular e-commerce platforms. The search usually leads to scientific suppliers—Sigma-Aldrich, TCI Chemicals, and Alfa Aesar. Sometimes, local niche distributors fill in the gaps, but large companies dominate requests in bulk. Lab-scale batches can look more like special orders, particularly in countries outside North America, Europe, or certain parts of Asia.
Price by Quantity and Use Case
In 2024, the market for N-Butyl-N-Methylpyrrolidinium Acetate isn’t as transparent as for basic lab solvents. If you need a few grams or just a small bottle, you’re likely looking at $150 to $300 per 10 grams depending on the purity and the vendor. Orders of 100 grams to a kilogram draw pricing down, though not dramatically. The price rarely dips below $1,000 per kilogram even for university researchers in my network, and most project managers I know consider it a higher-cost ingredient. That matches the experience of most researchers who have tried to source it for work with ionic liquids in biocatalysis or advanced polymer work.
The exact price reflects not only supply and demand but the headache of shipping regulated chemicals, regional customs, and paperwork. One time, my team tried to buy a similar imidazolium derivative and we spent more time sorting import certifications than running experiments. Extra costs pile up out of nowhere: documentation, surcharge fees, shipping. This holds true for pyrrolidinium compounds, too, and that’s before factoring exchange rates or fuel surcharges.
Is This Compound Hard to Get?
Trying to find reliable, consistent sources for specialty ionic liquids shows just how uneven chemical supply chains remain. Distributors sometimes quote weeks for lead time, referencing batch production or limited stock. I’ve spoken with chemists who ended up working with local syntheses. Most universities and corporate labs gravitate toward established supply channels—negotiating bulk prices or forming partnerships with vendors—since quality and documentation matter just as much as price.
Safety, Transparency, and E-E-A-T Factors
Safety matters a lot. Responsible buyers demand up-to-date safety data sheets, purity analytics, and verified supply routes to ensure nothing dangerous or fake sneaks in. With N-Butyl-N-Methylpyrrolidinium Acetate still relatively rare outside specialized labs, reaching out to multiple vendors can speed things up. Vetting suppliers through peer references or supplier review platforms saves time and headaches. In my own work, I’ve emailed technical reps before placing any order—getting lots of clarity over stability, degradation risk, and storage quirks.
In practice, only trained professionals should handle this salt, especially if scaling up experiments. For readers with a home chemistry interest, legal and safety barriers make casual purchases impractical. I’ve seen projects stalled or canceled after customs flagged an incoming order, so plan ahead, budget extra time, and document each step.
Potential Solutions to Common Obstacles
The path to reliable supply starts with clear planning. Build relationships with reputable chemical suppliers, and ask early about volumes, documentation, and compatible shipping carriers—this avoids surprises later. If delays hit, seeking out local academic partners or consortia sometimes opens new supply lines. Open science forums and research networks present another route, as peer labs sometimes have surplus stock or shared contacts.
No solution fits every budget or time frame, but moving early, communicating directly, and checking paperwork at every step makes the process smoother. For anyone depending on specialty chemicals, these habits become second nature.