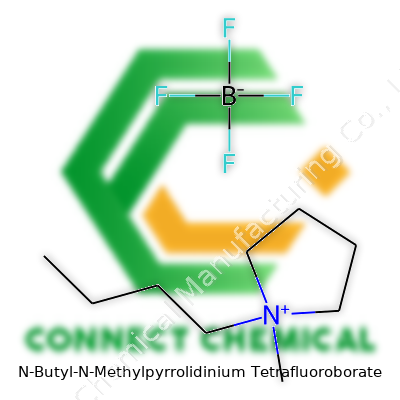
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate: More Than Just an Ionic Liquid
Historical Development
The story of N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate (or BMPyrr BF4, as chemists often call it) tracks alongside the broader arc of ionic liquids over the last forty years. Back in the 1980s and 1990s, chemists searching for smarter solvents started experimenting with molecules that could stay liquid at room temperature, and that wouldn’t evaporate like old-school solvents. BMPyrr BF4 grabbed attention because of its stable pyrrolidinium backbone and non-volatile tetrafluoroborate anion. By the early 2000s, research groups in Japan and Europe had started publishing applications for BMPyrr BF4 in everything from electrochemistry experiments to green chemistry initiatives, showing off qualities that regular organic solvents just couldn’t match.
Product Overview
BMPyrr BF4 sits among a family of ionic liquids that don’t run into the common pitfalls of flammability or narrow liquid range. As a room-temperature ionic liquid, this compound handles a surprising number of jobs that used to need harsh chemicals. Industry and academia alike jumped at the chance to use something with a relatively simple preparation route, less hazardous waste, and better chemical stability. As far as I’ve seen in labs or read in tech sheets, availability in high purity and at commercial scale made a real difference for its adoption in sensitive applications, especially since cross-contamination and water uptake can really throw off results.
Physical & Chemical Properties
Looking into its actual properties, BMPyrr BF4 remains colorless or just faintly yellow in appearance, with a viscosity that almost feels like thick oil at room temperature. It doesn’t freeze until temperatures dip far below zero, and it boils at extremely high temperatures because the ions don’t want to let go of each other. Electronegativity shifts from the BF4 component translate to good electrochemical stability, ideal for use as an electrolyte material. Measuring conductivity shows numbers higher than many older ionic liquids. Hydrophobicity keeps water mostly out, but once some gets in, special storage and handling become necessary—anyone working in synthesis or battery development learns this pretty quickly.
Technical Specifications & Labeling
Manufacturers ship the compound under straightforward naming conventions and ensure batch-to-batch consistency through rigorous trace impurity testing (think water, halides, transition metals). Labels list purity (often 99%+), melting point (below -20°C), and electrical conductivity data. Proper labeling also includes shelf life information, safety measures for skin and eye contact, and instructions on protective storage away from light and humid air. These details aren’t just legal hoops to jump through—they keep research and industrial outcomes reliable.
Preparation Method
Synthetic routes for BMPyrr BF4 tend to start with N-methylpyrrolidine and n-butyl halide, reacted under solvent-free or mild conditions to avoid unnecessary by-products. After formation of the pyrrolidinium salt, the halide exchange with sodium tetrafluoroborate brings in the BF4 anion. Purification involves rigorous washing and vacuum drying to weed out water and halide residues: missed cleaning can wreck downstream performance, especially in devices like supercapacitors or electrochemical sensors. Labs and manufacturers continue to look for easier and less waste-intensive pathways, since demand for greener processes never really lets up.
Chemical Reactions & Modifications
Researchers use BMPyrr BF4 not just as a solvent but as a reaction partner in some ionic exchange and separation processes. The cation’s structure can be swapped out with other alkyl groups through fairly routine organic reactions, but the core pyrrolidinium framework stands up well to various forms of stress, whether temperature swings or exposure to oxygen. This makes it a favorite for modification experiments, including introducing small amounts of functional groups for task-specific ionic liquids. The BF4 anion, while notorious for hydrolysis under very wet conditions, generally sticks around for most synthetic applications.
Synonyms & Product Names
Besides ‘N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate’, this compound appears on shipment manifests and catalogs as BMPyrr BF4, 1-butyl-1-methylpyrrolidinium tetrafluoroborate, or simply [BMPyrr][BF4]. Some suppliers shorten the name even further for logistical convenience. Spotting these variants on shipping manifests always helps avoid mix-ups, especially when working in labs with multiple ionic liquids on hand for different tests.
Safety & Operational Standards
Handling BMPyrr BF4 requires respect for its chemical profile: safety goggles, gloves, and lab coats protect against accidental splashes or spills. The compound shows low flammability, making it less hazardous than many traditional solvents, but direct skin contact brings some risk, as occasional irritation may pop up. Ventilation becomes important in larger-scale operations to avoid airborne particles. Any waste needs classification under local hazardous regulations due to possible environmental persistence. Having spent time working in university labs, I’ve seen even small lapses in these precautions create headaches during audits or routine inspections, impacting project timelines and trust.
Application Area
Early papers pointed toward electrochemistry as the main playground for BMPyrr BF4, largely because of its wide electrochemical window and ionic conductivity. Those developing batteries, supercapacitors, or even solar cells leaned toward it as a replacement for volatile organic electrolytes. Today, chemists use it in metal plating, protein separation, and even in 'designer' solvents for difficult-to-dissolve molecules. The push for greener chemistry breathed new life into BMPyrr BF4’s applications, since reusability and low vapor pressure align well with safety and sustainability targets. I’ve watched it pop up in patent filings for industrial lubricants and novel reaction media, where stability under stress reigns supreme.
Research & Development
Academic and corporate labs keep searching for new tweaks—adjusting alkyl chain lengths, blending with other ionic liquids, or pairing BMPyrr cations with different anions for specific properties. One fast-moving direction involves tailoring the solvent environment to stabilize reactive species, opening doors in organic synthesis and separations. Cross-disciplinary research welcomes input from physical chemists, electrochemical engineers, and materials scientists, all trying to move beyond incremental gains. Expansion in computational chemistry accelerates the pace, since researchers can model property changes in silico before making costly batches.
Toxicity Research
Although BMPyrr BF4 ranks lower on the toxicity scale compared to some fluorinated chemicals, published studies point out potential downsides if large quantities enter water systems. Chronic effects on aquatic organisms and slow breakdown in soil and sediment highlight the importance of containment, disposal, and avoidance of unnecessary releases during industrial processes. The compound’s low volatility limits inhalation risks but brings attention to possible skin absorption. Responsible labs run routine toxicity checks and stay alert to updates in global chemical legislation. I’ve found that risk perception can vary—some colleagues relax their guard because of the ‘low hazard’ label, but safe habits matter for the cumulative impact.
Future Prospects
Every year brings calls for safer, more energy-efficient, and less wasteful industrial protocols. BMPyrr BF4’s resilience and adaptability ensure it will stick around, especially in next-generation batteries, high-performance lubrication, and chemical separations. Moves toward scale-up, improved recycling, and closed-loop systems attract attention from governments and corporate sustainability teams alike. Research continues in developing bio-based alternatives, although BMPyrr BF4 still holds a strong lead in electrochemical stability and processing compatibility. Its future hinges on ongoing improvements in lifecycle analysis, regulatory oversight, and commitment to safe, responsible deployment in both established industries and new tech frontiers.
Behind the Name—What It Does
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate doesn’t sound like something most folks run into every day—but in labs and certain industries, it plays a quiet but vital role. This compound is what chemists call an “ionic liquid,” a salt that stays liquid at room temperature. That feature gives it a few superpowers that many other chemicals on the shelf just can’t match.
Batteries, Clean Energy, and the Push for Better Tech
Look at the guts of a modern battery, and you’ll see a whole chemistry set working together. This ionic liquid has found a place in lithium-ion and next-generation batteries since it doesn’t catch fire or evaporate easily. Traditional electrolytes in batteries can run into safety issues—spilling flammable solvents and breaking down when things get hot. N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate stays stable, which can reduce risks of fire or leaks.
I’ve seen this firsthand in some research labs—engineers can stress-test new batteries, pushing them harder without worrying about the whole thing turning into a hazard. It makes room for safer products in electric cars, consumer devices, and even future grid storage.
Green Chemistry and the Drive to Ditch Toxic Solvents
Plenty of news covers plastic pollution and toxic waste piling up. Manufacturing has relied on harsh solvents that poison waterways and threaten workers’ health for decades. Here’s where this ionic liquid stands out: it replaces older, more dangerous chemicals in several industrial processes.
As a solvent, it can dissolve both organic and inorganic stuff—a rare trick—so chemists use it to run cleaner reactions and recycle valuable metals from electronic waste. Large chemical plants, especially in places with strict environmental rules, can cut nasty emissions and create less waste water. Just a couple of decades ago, swapping toxic solvents for something safer seemed out of reach. Now, with compounds like this one, cleaner production isn’t just wishful thinking.
The People Factor—Practical Gains and Tradeoffs
Science has a human side, too. Lab safety improves—less inhalation risk, fewer burns, simpler storage. Workers gain peace of mind, and smaller companies manage costs because these liquids don’t evaporate or demand expensive ventilation. The bonus? Less money and energy wasted replacing lost chemicals.
Still, nothing arrives without tradeoffs. While N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate is more stable, it doesn’t break down quickly in nature. Long-term, we’re still figuring out the safest ways to recover and reuse it. Regulators and researchers need clear guidelines and transparency about how these substances move through soil, water, and air. Community input matters, especially in neighborhoods near manufacturing hubs.
Next Steps for Industry and People
Safer batteries and cleaner manufacturing feel urgent as the world eyes energy transformation and lower pollution. Progress depends on more than chemistry—it takes open data sharing, strict supply chain checks, independent testing, and regular health monitoring around factories. Community leaders get involved, making sure policy keeps pace with science.
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate won’t show up in your kitchen or backyard, but its impact touches daily life—better tech, safer jobs, and new ideas in green chemistry. These gains show that even obscure molecules can shape the future, right down to our homes and environment.
Getting a Grip on the Physical Side
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate doesn’t look all that exciting at a glance. You’ll find it as a clear to slightly yellowish liquid, easy to pour, and not a whiff of a strong smell. The story gets interesting around room temperature—this stuff stays liquid beyond where water would freeze solid, giving folks working in cold labs a breather. Iced up reagent bottles aren’t a problem with this one. Density feels hefty in your hand, thanks to the presence of those big ions packed tightly. Viscosity runs high, almost syrupy at lower temperatures, so it lags behind regular water if you try to swirl a beaker.
Solubility shifts depending on what you toss it in. Water barely touches it—it’ll give a milky look, but not much happens. Mix N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate with acetonitrile or other polar organic liquids, and it slides right in. In the lab, this means selective mixing becomes possible. Folks working on battery electrolytes or green solvents like having this sort of control, as it opens fresh doors that water-based solutions slam shut.
Chemical Personality: What Sets It Apart
Being an ionic liquid, this compound refuses to behave like salt you shake over fries. The ions stay fully split at room temperature. What blows me away is the stubborn stability. You can heat this up to over 200°C, and it barely bats an eye. Decomposition won’t ruin your day under regular conditions.
It doesn’t burn—a serious boon for anyone who’s ever watched a flammable solvent bottle sweat in the heat. No flashpoint to worry about means storage feels a lot less stressful. The downside? Get any water in there and you’ll trigger hydrolysis; that’s a word for slow but sure breakdown, which you want to avoid, especially if you’re after pure results in fine chemical work.
Chemically, it remains nonreactive with the usual suspects like glass and plastics, which makes it a go-to for electrochemistry setups and custom reactors. The tetrafluoroborate anion holds up well unless exposed to strong acids or bases. I’ve seen folks run fancy synthesis tricks using this stuff as a solvent, taking advantage of how it stabilizes charged intermediates—a bonus that lets them dial in selectivity and yields.
Why It Matters and How to Tackle the Challenges
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate delivers more than some niche chemical curiosity. In my work with batteries, using solvents that don’t light up like a bonfire changes the safety landscape. The risk drops, both for folks running big battery plants and labs that can’t afford even one fire.
From the green chemistry lens, this compound nudges us toward less waste and lower volatility. Its low vapor pressure cuts down on air emissions—important when you think of how many solvents evaporate under our noses. On that note, handling still matters. Even low-toxicity ionic liquids need proper containment. Gloves and goggles remain the rule. Production scale brings up new questions; waste disposal and costs often spike with specialty chemicals.
Looking to the future, success hinges on recycling and recovery techniques that can handle ionic liquids without breaking the bank. The chemistry world keeps pushing for more robust, less demanding production cycles. I remember the headache of disposing of mixed solvents after a long week at the bench. N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate hints at a shift—reuse, recycle, and smarter process design. Advances here could make labs and factories quieter, cleaner, and a whole lot safer.
Understanding What You’re Handling
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate, a mouthful for sure, pops up in electrochemistry circles and battery labs. Often called an ionic liquid, it has a few key qualities: low vapor pressure, decent thermal stability, and ability to dissolve all sorts of stuff. But the safety questions don’t just fly away because it looks tame on the surface.
Why You Should Care About Safety
Talking to professionals in chemistry labs, nobody shrugs off safety data sheets. Even though this compound doesn’t flash up like gasoline, it brings worries of its own. Skin contact might irritate. Splashing into eyes usually does not end well. Breathing vapor or dust—even at low levels—rarely improves your day. Some folks believe ionic liquids are almost harmless, but smaller molecules don’t get all the attention because bigger risks steal the spotlight.
Anything with a tetrafluoroborate group deserves respect. Tetrafluoroborate can break down and produce hydrofluoric acid, which no one wants near their hands or lungs. HF burns don’t look serious at first, but they wreck tissue and can lead to deep health issues. The risk goes up if you heat things up or mix with water or strong acids. That gives enough reason to step up your protective routine.
Personal Protective Equipment Makes the Difference
Gloves, lab coats, and goggles should never be optional. I’ve seen too many smart people shrug off gloves and pay for it with red skin and a trip to the nurse. N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate calls for chemical-resistant gloves, not those thin latex ones, because leaks and pinholes in disposable gloves let more through than you’d think. Eye protection shields against accidental splashes—nobody feels like rinsing out their eyes at an eyewash station because they skipped goggles just once.
Using this compound inside a fume hood keeps the vapors out of your lungs and the room. Even if vapor pressure stays low, spills or heat can push out enough fumes to bite back. Ventilation always beats guessing what might linger in the air. Lab SOPs exist for a reason, and staff should know the quickest route to safety showers and first aid. Training is not just formality; muscle memory counts in a panic.
Environmental and Disposal Responsibility
I learned the hard way that you can’t wash this stuff down the drain. Regulations treat waste ionic liquids pretty seriously, with good reason. Some breakdown products hurt aquatic life or poison water systems. Sealed containers and hazardous waste disposal channels take care of the leftovers. If your lab skips these steps, someone winds up in trouble with regulators and the environment pays the bill.
Risk Reduces with Solid Habits
Reviewing safety data before pulling any chemical off the shelf builds good habits. Working with a buddy, keeping spill kits on hand, and storing N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate in sealed, labeled containers helps everyone in the lab. It doesn’t take fancy tech—just common sense and respect for chemistry that can turn on you if you look the other way.
Possible Solutions for Safer Handling
Switching to less hazardous ionic liquids can cut down on some risks, but that’s not always an option. Encouraging strong culture around PPE and spill management keeps people safer every day. Regular safety drills mean muscle memory stays sharp. Updating safety information as new data comes out stops surprises before they start. Respect for chemical hazards comes from experience and mindfulness, not just printed protocols. Doing the basics right saves skin, eyes, and sometimes even lives.
Industry’s Benchmark for Purity
In labs that handle ionic liquids and advanced electrolytes, N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate isn’t just a mouthful—it’s a real workhorse. Over the years, I’ve watched researchers and battery engineers reach for this salt when they have to balance high ion conductivity with chemical stability. The first thing anyone checks before buying or using it? Purity. A solid lot of commercial bottles list a purity at 99%. Every percentage point below that signals a few more headaches for someone down the line. Researchers using anything under 99% often run into sluggish reactions, noisy electrochemical data, and sometimes, outright cell failure.
People sometimes think a single percentage point can get lost in the mix—truth is, it doesn’t. Small traces of water or other organic impurities shift voltammetric windows or help corrosion kick off, especially in moisture-sensitive battery work. Water content below 100 ppm is usually demanded, and producers use Karl Fischer titration to verify. Some reputable producers publish impurity profiles showing halide, alkali, or trace metal contents each under 1–5 ppm, because lab directors have memories like elephants when a cell blows up because of careless impurities.
Why These Specifications Matter
Knowing what you’re actually getting is crucial. Take viscosity. In the best samples, the viscosity reads between 75–110 centipoise at 25°C, which determines whether you’re prepping an experiment or unclogging pipettes. The ionic conductivity should hover around 3–7 mS/cm, and any off-spec value warns of funny business, either storage or synthesis routes gone wrong. This isn’t a hypothetical concern. A few years back, one lab I worked with shelved a whole series of results because an supplier’s batch, labeled 98%, had too much residual chloride, and the conductivity dropped by half.
Color is a good tell. A decent batch stays clear and nearly colorless—any yellow or brown cast shows aging or impurity buildup. Storage makes a difference. The best manufacturers pack it in airtight glass, under dry nitrogen, because open air turns shelf-stable electrolytes into expensive paperweights. That critical dryness means packaging is just as important as the bottle’s label.
People Overlook Details: Risks and Ways Forward
Product data sheets only get you so far. If you’re running heavy-duty synthesis or electrochemical work, dig deeper—ask for a certificate of analysis, not just a catalog page. Compare results batch-to-batch, especially if you change suppliers. Scale-up work only exposes more ways for contamination to creep in, so staying vigilant with pre-use quality checks keeps projects on track.
It helps to align with suppliers who run regular residual solvent and volatile analyses, and verify with your own NMR and ion chromatography, if possible. Regulations around handling and disposal are catching up. Not enough projects plan for waste streams containing tetrafluoroborate, and municipalities are starting to step in, demanding proof of safe containment and neutralization.
Factoring In Trust and Transparency
The future for high-performance ionic liquids such as N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate hinges on trust—with suppliers producing trustworthy, thoroughly characterized chemicals, and users double-checking, not just assuming the spec sheets tell the full story. Researchers and companies taking these steps stand a better chance at reliable data and safer, more robust tech.
Why Proper Storage Matters
N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate has carved out a spot in electrochemistry labs, battery research, and industrial chemistry. I’ve seen this compound sparking conversations for its surprising thermal stability and low volatility, which sounds like peace of mind for storage. But without careful handling, unexpected issues like moisture uptake and degradation can creep up and spoil entire batches. A ruined stock means wasted funds and disrupted research, and nobody wants that frustration mid-project.
A Dry, Cool Place: Not Just a Cliché
In most labs I’ve visited, there's a cabinet for chemicals like N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate—dark, dry, and always labeled. This isn't just about habit. Research shows this ionic liquid reacts with ambient moisture. Even a slight water uptake starts breaking down its structure over months, changing its conductivity and purity. I once handled a batch that sat out after the cap was left loose; we measured water content a week later, and values had doubled, making it useless for precise measurements.
So, storage in tightly sealed glass bottles, preferably amber-colored, protects against both light and air. I prefer glass over plastic since some plastics leach and change the liquid’s composition. A desiccator cabinet with silica gel works best in humid climates. For anyone with limited resources, a plastic bag with desiccant in an office drawer beats a shelf in an open-air room. Every bit of moisture you block extends shelf life.
Temperature: Stay Below Room Heat
This chemical can handle a range of temperatures, but room temperature storage—between 15°C and 25°C—avoids crystallization and decomposition. Keeping temperatures stable makes life easier for the next person who needs a reliable reagent; nothing’s worse than pulling a viscous, unusable sample during crunch time. Don’t freeze it, either. Some ionic liquids face separation or precipitation when pulled from a cold to warm state, and you risk irreversible changes.
Shelf Life: Trust Your Label, Then Test
Manufacturers suggest two to three years of shelf life if the bottle stays sealed, dry, and away from direct sun. Opened bottles can last 12 to 18 months if you’re diligent with sealing and desiccants, based on published chemical stability data. But I never trust a number blindly. If purity or conductivity are critical, I pull a small sample and test before committing it to a big experiment. Small impurities can throw off an entire battery prototype or electrochemical assay.
Solutions for Safe and Long-Term Use
Some simple habits go a long way. Always label bottles with the date opened. Rotate older stock to the front, just like milk in a fridge. Use desiccant packs and swap them out if they start changing color from water absorption. If you're in a place with frequent storms or blackouts, consider a backup storage area with climate control. I once lost a batch because of an air conditioning failure in the middle of summer—humidity soared and ruined it in days.
Education for new lab staff pays off too. I walk through correct handling with every round of new students. Investing in a few reusable desiccator boxes or humidity indicators doesn’t break the budget and saves trouble down the line.
Well-handled N-Butyl-N-Methylpyrrolidinium Tetrafluoroborate rewards you with consistent, repeatable chemistry—just don’t take shortcuts, and your lab will thank you later.