N-Butyl-N-Methylpyrrolidinium Trifluoroacetate: A Deep Dive into its Rise and Impact
Historical Development
Talking about N-Butyl-N-Methylpyrrolidinium Trifluoroacetate is like tracing the story of ionic liquids and their place in modern chemistry. Ionic liquids were once a curiosity, stuck in academic circles, far from real-world applications. Chemists have known for decades that organic salts could remain liquid at room temperature, but it took leaps in materials science through the 1990s for compounds like N-Butyl-N-Methylpyrrolidinium Trifluoroacetate to take shape. Researchers reached for these substances while searching for solvents that sidestep the volatility and toxicity problems of traditional organics. As interest grew in green chemistry and safe alternatives, labs worldwide started to include pyrrolidinium-based compounds in their pursuit of safe, efficient solvents. Today, thanks to persistent research and collaboration across universities and industries, these ionic liquids have moved beyond theory and patents and landed on the bench tops of chemists working in energy, catalysis, and synthesis.
Product Overview
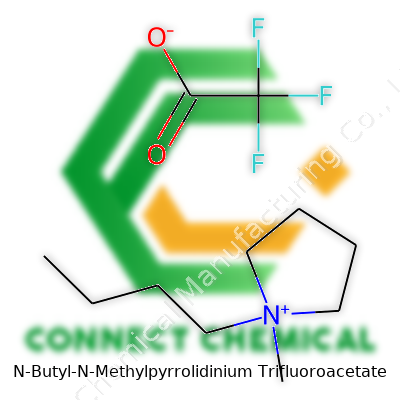
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate stands out as an ionic liquid, holding unique advantages compared to classic organic solvents. It combines a pyrrolidinium backbone—rigid, reliable—with the strong, non-coordinating trifluoroacetate anion. The resulting compound resists water uptake and maintains thermal stability, allowing for smooth use in sensitive reactions. Companies now produce it for researchers who want less volatility in the lab and a broader temperature window. It fits the bill for scientists handling temperature-sensitive reactions or trying to cut waste by limiting solvent evaporation. From my perspective in academia, getting samples with reliable purity and detailed labeling has become easier each year, a true shift from the early days of niche, hard-to-source compounds.
Physical & Chemical Properties
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate often draws interest because it’s a colorless to pale yellow liquid that stays stable and manageable at room temperature. Its low vapor pressure means fewer headaches over harmful emissions or solvent loss, which has a certain comfort, especially in labs with limited fume extraction. Chemists respect its thermal stability; the compound resists decomposition at temperatures that send many organics gasping for air. Its ionic nature brings high conductivity—a major plus for anyone working in electrochemistry. Its viscosity tends toward the higher end, which sometimes calls for balancing speed of mixing with reaction control. The trifluoroacetate anion gives acid-base chemistry a twist, letting users modulate reactivity by tweaking local environments. Though it mixes well with other organics and some water, it pushes back against hydrolysis, holding its form even in damp air.
Technical Specifications & Labeling
Producers of N-Butyl-N-Methylpyrrolidinium Trifluoroacetate understand that detail matters. Bottles arrive with transparency on purity—usually greater than 99%—with residual water and trace metal content spelled out for buyers. Detailed spectra (NMR, MS, IR) accompany each batch, helping buyers build trust. Labels note the CAS number, chemical formula (C11H18F3NO2), and even storage instructions to keep the compound away from strong acids or bases. I remember my first order of such a specialty compound: facts on labeling let us set up experiments without guessing about shelf life or contamination risk. That transparency supports safety and replicability—cornerstones of any trustworthy supply chain.
Preparation Method
Chemists prepare N-Butyl-N-Methylpyrrolidinium Trifluoroacetate through careful alkylation and subsequent ion-exchange reactions. The first step usually joins an N-methylpyrrolidine base with a butyl halide, often under nitrogen to dodge side oxidation. This forms the cation, which is then met with trifluoroacetic acid or a trifluoroacetate salt, completing the transformation. Every step emphasizes exclusion of water and oxygen, as side reactions can create stubborn impurities. Careful distillation and repeated washing leave a product ready for research or industrial use. Custom tweaks in process—such as slower addition rates or alternate solvents—adjust yield or viscosity, serving projects that need large volumes or tailored reactivity.
Chemical Reactions & Modifications
Researchers use N-Butyl-N-Methylpyrrolidinium Trifluoroacetate in an array of chemical settings, appreciating its resistance to nucleophilic attack and oxidation. The cation, anchored by the pyrrolidinium ring, tolerates tough conditions without fragmenting—a feature valued in electrolysis or catalysis. Swapping the trifluoroacetate with related anions creates a family of ionic liquids, each with distinct acidity, hydrophobicity, or solubility. Such flexibility lets scientists pick a solvent fit for a job, not just settle for what’s on the shelf. Functionalization of the cation or anion also tunes performance in battery research or biomass processing. From my own work, these modifications often become the heart of collaboration with synthetic teams, who can tailor-make fluids that simplify purification or product isolation.
Synonyms & Product Names
This compound carries several names, reflecting the conventions of organic nomenclature. Chemists may spot it listed as 1-Butyl-1-methylpyrrolidinium trifluoroacetate, BMPyr-TFA, or, in catalogs, as a pyrrolidinium-based ionic liquid. Trade names from suppliers sometimes shorten or stylize the labels, but the molecular formula never changes. Recognizing these variants helps anyone navigating suppliers, safety sheets, or published data without confusion.
Safety & Operational Standards
Laboratories using N-Butyl-N-Methylpyrrolidinium Trifluoroacetate treat it with the respect due any strong organic chemical. Even though it sheds the volatility of ether or acetonitrile, it poses real risks if handled carelessly. Users wear gloves—nitrile provides protection—and splash goggles, especially since ionic liquids can show skin or eye irritation. Fume hoods stay open during weighing or transfer. Storage calls for cool shelves out of direct sun, and far from both acids and oxidizers. Spill kits with absorbents made for ionic liquids close the loop for safe cleanup. Producers publish up-to-date safety data sheets, noting LD50 information, symptoms of exposure, and waste disposal routes. I’ve trained undergraduate lab members on its handling, stressing that “greener” solvents only reduce, never eliminate, lab hazards.
Application Area
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate appears in places where old solvents can’t keep up. Batteries and fuel cells welcome its stability and high ionic conductivity. Synthetic chemists mix it with catalysts to unlock reactions that struggle in water or volatile organics; it simplifies separation of products through selective solubility. Bioprocessing companies reach for it to break down biomass or extract rare compounds without resorting to harsh, outdated solvents. In my experience, its reliability in electrochemical setups reduces maintenance downtime, helping research teams focus on results rather than fixing leaks or scrubbing corrosion. Its use has expanded into pharmaceuticals and analytical chemistry, where selective extraction without cross-contamination has real value.
Research & Development
The story of this compound doesn’t stop at current applications. Universities and private companies keep searching for ways to get more performance—greater stability, lower viscosity, lower cost—from pyrrolidinium ionic liquids. New derivatives, coupling the parent structure with functional groups that target proteins, metals, or other ions, look promising for tailored extraction or sensing. Open data sharing and partnerships make for quick progress, and attending conferences I see more and more posters each year focused on these ionic liquids. Funding from groups interested in clean tech and sustainable chemistry pours fuel on the fire, ensuring this area will keep evolving.
Toxicity Research
While ionic liquids rank as safer than many classic organics, toxicity remains a central question. Animal studies and cell assays show that while pyrrolidinium-based fluids outclass imidazolium relatives for biocompatibility, care still rules the day. Acute exposure can trigger skin or mucous membrane irritation; oral or inhaled doses at high levels trigger systemic effects. Long-term studies remain scarce, though—they need funding and strict controls. Waste management threads through every experiment, with waste containers marked and tracked according to both chemical and potential biological hazard. Recent reviews on ionic liquid toxicity urge us to design with greener exits in mind, such as developing products that degrade harmlessly after use. In my labs, students keep exposure logs and double check mercury and heavy metal contamination before disposal, keeping the research side of green chemistry honest.
Future Prospects
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate stands poised to stretch its influence deeper into sustainable chemistry. As energy storage moves ever closer to practical ion-based devices, this compound’s stable window and reliable conductivity offer a convincing answer to performance bottlenecks. Synthetic routes keep simplifying, dropping both cost and waste. Each year, new applications appear: enzyme stabilization, low-temperature extractions, recovery of rare earths. The next advances will depend on deeper understanding of structure-function relationships, letting designers tune the balance of hydrophobicity and reactivity for each setting. I see opportunities not only in the lab, but in full-scale systems—chemical plants, recycling facilities, even field labs in resource-starved settings. Responsible stewardship, open research, and honest conversation between industry and academia will set the pace, making sure that innovation comes with sound safety and sustainability.
Why Chemists Pay Attention to N-Butyl-N-Methylpyrrolidinium Trifluoroacetate
N-Butyl-N-methylpyrrolidinium trifluoroacetate isn’t a term you hear at the grocery store, but scientists and engineers have gotten pretty familiar with it over the past decade. This is an ionic liquid — a type of salt that stays liquid under normal conditions. That property unlocks a lot of possibilities that go far beyond traditional laboratory solvents. I’ve worked on projects where solvents failed us, either because they reacted too easily or simply evaporated, and finding a more stable substitute sometimes meant better data and safer labs.
A Safer, More Flexible Solvent for Research
Folk often turn to this compound in laboratories trying to avoid the dangers of volatile organic solvents. Traditional options like acetone, toluene, or chloroform often stink up the room and pose fire risks. N-Butyl-N-methylpyrrolidinium trifluoroacetate, with its low vapor pressure, barely evaporates at room temperature. You can run reactions in open air, and there’s less risk of breathing in toxic fumes. I’ve noticed labs switching to this and similar ionic liquids because students don’t want to worry as much about health or safety hazards that give departments legal headaches.
Boosting Efficiency in Catalysis
Ionic liquids have shaken up the world of chemical synthesis. With this particular compound, chemists can dissolve a wide range of substances that don’t mix well in water or oil. In catalytic reactions — essentially the chemical world’s version of speed-boosts — this salt stabilizes catalysts that would normally degrade or clump up in water. Some teams focused on green chemistry tout it because you can often recover and reuse both the ionic liquid and the catalyst, cutting waste and slashing costs. These days, sustainability gets more than a passing mention during funding meetings, so this practical advantage makes a difference.
Separating Materials with More Precision
Separation is a big part of making chemicals, pharmaceuticals, and even some food ingredients. Engineers have invested years into getting better at pulling one substance from a mix of others. N-Butyl-N-methylpyrrolidinium trifluoroacetate’s mix of properties — it’s polar, it dissolves a huge range of molecules, and it keeps stable under heat — gives it a leg up for use in extraction and purification systems. Take cellulose extraction, for example: using ionic liquids, teams can process plant matter for applications ranging from new materials to fuels. Without the right solvent, you can’t break down the tough bonds in plant fibers. I’ve seen groups move from harsh acids to these ionic liquids, ending up with less equipment corrosion and higher yields.
Looking Toward Greener Tech
Science has an environmental problem once production scales up, and many academics and startups are on the hunt for cleaner chemical tools. N-Butyl-N-methylpyrrolidinium trifluoroacetate draws attention from the green chemistry crowd mainly because it often replaces volatile or toxic liquids. Nobody wants to hear about another chemical spill, and replacing common hazards with something more stable seems like an obvious way forward. But ionic liquids like this still raise questions — what happens if they escape into waterways, how expensive are they to recycle, do they stick around in the environment too long? Regulators and industry leaders need better long-term tests and rules before scaling up further.
More than a Lab Curiosity
A single chemical doesn’t fix every problem facing manufacturing or research, but switching old solvents for N-Butyl-N-methylpyrrolidinium trifluoroacetate could make real differences. The labs I’ve worked with valued the adaptability and safety this salt brought to their research benches. As technology keeps advancing, I expect to see more attention on both what these chemicals can do — and how we handle them safely.
Unpacking the Formula
Researchers and chemists often run into N-Butyl-N-Methylpyrrolidinium Trifluoroacetate. It pops up in labs focused on green solvents, advanced batteries, and even pharmaceutical development. To break it down, the chemical formula for N-Butyl-N-Methylpyrrolidinium Trifluoroacetate is C11H20F3NO2. Building it up, you get C7H16N from the N-butyl-N-methylpyrrolidinium cation, and C2F3O2 from the trifluoroacetate anion. Stick these together, and you end up with a structure that brings together a five-membered nitrogen ring, a methyl group, a butyl chain, and a negatively charged anion loaded with three fluorines and two oxygens.
Molecular Weight in Practical Terms
The molecular weight helps chemists measure reagents efficiently, track yields, and predict reactivity. Here, N-Butyl-N-Methylpyrrolidinium Trifluoroacetate comes to around 255.28 g/mol. This looks like a dry number on the page, but anyone who has weighed out a compound at the bench knows molecular weight drives everything from stoichiometry to separation methods. You just can’t skip over the basics—get that molecular weight wrong, and everything downstream can go sideways. I remember early mistakes in the lab, where wrong calculations led to heaps of wasted solvents and cleanup.
Why the Structure Matters in Real Research
This salt’s unique cation and anion open doors in several fields. The pyrrolidinium ring stabilizes the cation, allowing good solubility in polar and nonpolar solvents. In my time screening electrolytes for lithium batteries, salts like these offered thermal stability, wide electrochemical windows, and resistance to decomposition. The trifluoroacetate piece gives low nucleophilicity, which helps avoid unwanted side reactions. It feels rewarding running reactions that stay clean instead of generating a mess of byproducts.
Beyond batteries, I’ve seen these compounds play a big role in catalysis and extraction. The balance of organic and inorganic character lets researchers tune reactivity. People at the bench favor options that limit toxicity, volatility, and environmental impact, since green chemistry isn’t just a buzzword. In one green solvent project, ionic liquids packed with fluorinated anions replaced volatile organics, the lab smelled better, and the waste stream got a lot safer to handle.
Addressing Challenges and Moving Forward
There’s no silver bullet, though. These salts sometimes drive up cost and face limited scale-up, especially when fluorinated raw materials see price hikes. Sustainability comes into play, and making molecules in fewer steps with less hazardous byproducts stays front of mind. I’ve met teams tinkering with alternative anions or even bio-based cations, just to nudge the chemistry greener and cut reliance on petrochemical feedstocks.
At the end of the day, knowing the composition and properties anchors safer, smarter science. Chemists keep an eye on evolving regulatory guidelines, and finding ways to work with, not just around, these rules, leads to progress. Paying attention to structure, weighing out reagents correctly, and thinking through environmental impacts—these habits don’t just improve experiments, they add up over time, both in efficiency and safety.
Understanding the Chemical
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate doesn’t roll off the tongue, but it gets plenty of attention in chemical labs and industry research. Anyone who’s worked with ionic liquids knows how valuable this one can be, especially for greener chemistry and advanced battery work. Whether you’re prepping a bench for new experiments or fine-tuning procedures, smart storage cuts down on risks and preserves the investment.
The Human Side of Safety
My time working in university and small biotech labs showed that safe storage starts with understanding—not just regulations, but your workmates who need to grab a bottle at 8 a.m. When a chemical like this one comes in, everyone should check the safety data sheet before it’s opened. Acetates and pyrrolidinium salts don’t react wildly to air or water the way some other reagents do, but this particular compound draws moisture from the air and doesn’t belong near strong acids or open flames. Keeping it away from the sink and high-traffic spots is just common sense.
Temperature and Light Matter
A lot of chemicals don’t need much caretaking, but trifluoroacetates can lose punch if left out in the open. Once, I left a small vial on a windowsill overnight. Next day, it was slick and a bit clumpy, far from what I’d started with. This one reminds you that a cool, dry, dark place outperforms a sunlit corner. A chemical fridge works great, keeping temperatures consistent and taking away any stress over accidental heating. Make sure moisture stays out by capping containers tightly.
Containers You Can Trust
Glass bottles with thick screw-top lids still beat everything for long-term storage. While plastics can leach or warp, glass blocks out smells and doesn't react. Labeling really matters, too. Many people have made the mistake of reusing containers with old tape, but clear, print-out labels prevent headaches later. I like to add my own label on the back, too, with the date and my initials, so colleagues know whom to ask.
Avoiding Cross-Contamination
When chemicals crowd together on a shelf, mistakes creep in. Space is limited in smaller labs, but shelving that splits solvents, acids, and different salts cuts down on mix-ups. I picked up a habit from a careful technician: placing a little absorbent mat under bottles, just in case a lid sticks or a drip follows a pour. One leak can mean ruined chemicals and a long cleanup.
Training and Documentation
People sometimes forget that even with one rare bottle, training comes first. Every person using the chemical should know emergency spill prep and what to do if bottles break. Digital sheets stored on the lab desktop help here, as does a whiteboard checklist for monthly bottle checks. Expiry dates matter less with stable ionic liquids, but a glance at the color or clarity before each use helps.
Proactive Solutions
I’ve found that putting a checklist right near the chemical shelf makes people stop and think before grabbing or storing anything. Lockboxes come in handy when multiple people use a space, setting a level of accountability. Sometimes, buying smaller bottles means less exposure and fewer accidents. People don’t always think about these extra steps but adding them means better results and safer teams, every time.
Final Thoughts
Careful attention to storage details builds good habits for the long haul. Even small tweaks in habit or storage setups protect everyone’s hard work—as well as health—day in, day out.
Chemical Curiosity or Hidden Risk?
Chemists spend a lot of time around compounds that sound like tongue twisters. N-Butyl-N-Methylpyrrolidinium Trifluoroacetate is one of those chemicals that comes up in labs working with ionic liquids or in settings where unusual solvents have a place. Not many people outside a research setting know its full story. That’s part of what makes conversations about its safety important.
What My Years in the Lab Taught Me
I’ve handled plenty of solvents with names just as long. With each new bottle, I grew cautious. Safety data sheets aren’t just paperwork exercises; skimming over a hazard symbol can turn a regular day of pipetting into one you remember for all the wrong reasons. N-Butyl-N-Methylpyrrolidinium Trifluoroacetate is no exception. Every chemical deserves respect until proven otherwise—because even the ones that lack drama on paper often make up for it in other ways.
Safety by the Numbers
Ask most researchers about this compound and they’ll point toward its use as an ionic liquid. These chemicals have lower vapor pressures than old-school organic solvents like acetone or ether, which means less of them ends up in the air you breathe. That’s promising. The lack of strong fumes cuts down on headaches and the fire risk drops compared to some of the more volatile substances out there.
The trifluoroacetate part tends to make people cautious. Trifluoroacetate can break down to release trifluoroacetic acid—a compound that’s not a household name but is recognized for being tough on ecosystems and hard to remove from water. It sticks around, raising a big flag for environmental chemists. That persistent nature means careless disposal, even of something used by a handful of researchers, can add up over time. Labs heading toward “green chemistry” want to keep a close eye on what gets washed down the drain, and trifluoroacetate-containing substances demand real scrutiny.
Real-World Safety Concerns
Hazards come in different flavors. Acute effects—what happens if you spill it on your hand or breathe it in—share space with long-term impacts, like what happens to the world outside the lab walls. I’ve seen researchers reach for gloves and goggles, knowing well that just because a chemical doesn’t smoke or burn doesn’t mean it can’t harm your skin or eyes. While N-Butyl-N-Methylpyrrolidinium Trifluoroacetate isn’t as dramatic as a bottle of concentrated acid, its effects on skin and mucous membranes matter. Irritation can sneak up on you. Every bottle in the supply closet deserves the same basic routine: gloves, goggles, and, if you value your lungs, a fume hood nearby.
Toward Better Habits and Choices
Building trust in the science community means sharing honest experiences. Plenty of chemists learn from a stubbed finger or a splash in the eye—that’s a lousy way to learn. Manufacturers and suppliers put together safety data sheets for a reason, and nobody on the bench gets extra points for ignoring them. Training, from undergrads to seasoned PIs, should hammer home the point that less exposure leads to fewer bad days and mistakes are more likely when taking shortcuts.
The push for greener solvents doesn’t just mean chasing low emissions or paper values. It means finding options that don’t stick around forever in our water or soil, and it means listening as soon as environmental persistence pops up in a hazard assessment. Looking out for safer substitutes or designing waste treatment that neutralizes stubborn fluorochemicals could bridge gaps between research needs and lasting safety. Until then, respect stays high on the lab bench—just as it should.
What Scientists Look For in N-Butyl-N-Methylpyrrolidinium Trifluoroacetate
Walking into any modern chemistry lab, you’ll notice how much attention settles on purity. N-Butyl-N-Methylpyrrolidinium Trifluoroacetate isn’t just an ordinary salt; it’s a kind of ionic liquid shaping electrochemistry, green catalysis, and organic synthesis far more than most realize. The molecule must show up clean, consistent, and ready to perform as expected. Chemists typically insist on purity levels above 98%. This figure isn’t chosen at random—a handful of experiments can derail if trace contaminants sneak in, especially moisture or other ionic liquids. Even a small impurity can skew reaction yields, mess with reproducibility, or throw off analytical instruments.
Researchers have spent days chasing down pesky side-products because a bottle didn’t match the specification. That’s more than an annoyance; valuable time goes straight out the window. Reputable suppliers sell this compound with written guarantees and HPLC, NMR, and elemental analysis sheets. The best batches come in at ≥99% purity, verified with sharp peaks and neat spectra. It takes years in the lab to see how invaluable that level of cleanliness becomes.
Typical Appearance: More Than Meets the Eye
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate comes in as a colorless to pale yellow liquid. The real thing is more than a clear description. On the benchtop, the liquid has a slight viscosity. In room lighting, it hardly tints yellow unless a batch spent time exposed to heat or light—both known to nudge some color due to decomposition. Small changes catch the practiced eye; murkiness, harsh odor, or unexpected tint signal problems. In my time working with ionic liquids, clean batches handle like slightly thickened water—no floaters, no sediment, no cloudiness, no funk in the nose.
A transparent vial tells its own story. If the bottle looks off, whether it’s from water absorption or oxidative color change, people start asking questions about stability. Once, an off-color sample stalled a project for a week while we waited for repeat analysis and a replacement. Appearance always matters.
Quality Matters: Why Purity and Appearance Deserve Attention
Every missed reaction costs hours. Academics juggling tight budgets get hit harder, especially when repeat orders stretch out timelines. Factories setting up pilot-scale electrochemical cells need to know ionic liquids won’t add unpredictable factors. For both groups, small investments in high-purity material pay off. Every sigma becomes critical in electrochemical windows, viscosity, or solvation. Journal reviewers watch for purity declarations in publications, too.
Cutting corners by accepting less-than-pristine chemicals leads to confusion. Strong reporting habits—showing purity, appearance, batch data, and supplier—head off arguments and boost trust between labs.
Supporting Quality: Practical Steps Forward
Some labs still rely on certificates of analysis from suppliers. That helps, but running random in-house checks adds a safety net. Regular NMR or Karl Fischer titrations for water content, plus a common sense eye on appearance, keeps everyone honest. I once learned the hard way: the cost of a single bad batch outweighs a dozen spot tests. Bringing these habits into daily routines speeds up learning, saves money long-term, and helps everyone—industry and academia—get the results they need with fewer surprises.
N-Butyl-N-Methylpyrrolidinium Trifluoroacetate won’t win any beauty prizes, but its clarity and confirmed purity do more for progress than flashy labels or marketing buzzwords. Experience keeps showing that in chemistry, a little vigilance on quality saves a whole lot of trouble.