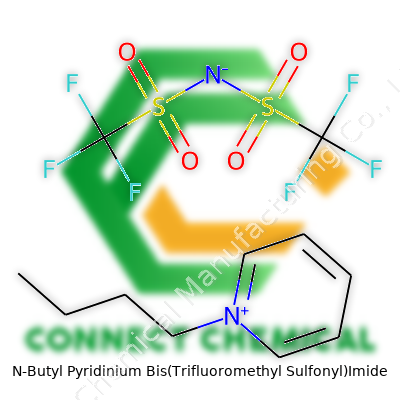
N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide: A Practical Look at An Evolving Compound
Historical Development
N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide has roots that stretch back to the late 20th century, during a period when chemists searched for alternatives to volatile organic solvents. Traditional solvents caused headaches for environmental regulators and exposed workers to risks. Ionic liquids like this compound answered the call by showing real promise — low vapor pressure, stability, and “green chemistry” potential. Academic labs in Europe and North America started synthesizing and publishing about pyridinium-based ionic liquids through the 1990s. The main draw was their thermal stability and tunable nature. Big chemical companies and start-ups began to license patents on ionic liquids and products containing these structures in the early 2000s, which only grew the market. Today, local labs and multinationals churn out countless variations, pushing this field far past its early experimental beginnings.
Product Overview
In today’s market, N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide carries the label of a room-temperature ionic liquid with specialized uses in electrochemistry, catalysis, and separation processes. Factories ship it in tightly sealed drums, often marked with hazard symbols that hint at its power and unique risks. What buyers look for is purity, which matters especially when working in battery or pharmaceutical settings. Import rules, handling protocols, and REACH registration status keep importers on their toes. Analytical reports usually include infrared and NMR spectra for assurance. Most researchers and engineers I’ve known want to see a consistent product, backed by third-party laboratory results, before trying it anywhere near a production line.
Physical & Chemical Properties
N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide shows up as a colorless to pale yellow liquid, known for its near-zero vapor pressure at room temperature. Its melting point usually lands well below room temp, and the viscosity can swing a lot depending on batch and purity. It stands out as highly dense and quite hydrophobic, rejecting water and easily forming slick, unmixed layers with most polar solvents. The structure owes a lot to the bis(trifluoromethyl sulfonyl)imide anion, which gives it low flammability and high electrochemical stability, often up to 4-5 volts, making it a favorite for next-generation battery research.
Technical Specifications & Labeling
Producers typically deliver this compound at purities of 99% or greater with residual moisture as low as 50 ppm. Labels must include hazard pictograms according to GHS guidelines, emphasizing the risk of skin and eye irritation. MSDS sheets supply details about decomposition products, toxicity, and safe disposal. Package sizes range from small vials for laboratory use to barrels for industrial settings. Accurate CAS numbers often appear on every bottle, and even the UN shipping codes show demand from regulated users who do not accept ambiguity on the label.
Preparation Method
Labs usually prepare N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide by quaternizing pyridine with butyl halide, producing N-butyl pyridinium halide salt. The next step swaps the halide with lithium bis(trifluoromethyl sulfonyl)imide in water or acetonitrile, using the low solubility of the final ionic liquid in water to drive separation. This counter-ion exchange allows for purification by washing and drying, followed by vacuum stripping to reach ultra-low water content. The process exposes technicians to lithium salts, so they often rely on fume hoods, gloves, and regular environmental checks. Most production lines must watch for halide contamination, which could disrupt both electrochemical and catalytic performance.
Chemical Reactions & Modifications
Chemical tinkerers have found ways to adjust both the cation and anion partners to tailor viscosity, hydrophobicity, and reactivity. Sometimes, they tweak the N-alkyl group length or substitute new functional groups. These changes can nudge the melting point up or down, or let the liquid dissolve new classes of organics and polymers. Some teams react the compound with metal salts to explore organometallic catalysis or recover precious metals from mixed waste. The bis(trifluoromethyl sulfonyl)imide anion holds up under strong reducing and oxidizing conditions, which means it can play well in radical chemistry or high-voltage battery configurations without breaking down.
Synonyms & Product Names
The most common names floating around in technical literature and chemical catalogs include N-Butyl Pyridinium NTf2, [BuPy][Tf2N], and 1-Butylpyridinium bis(trifluoromethylsulfonyl)imide. Some suppliers abbreviate the anion as [NTf2] or [TFSI] and the cation as [BuPy]. Older catalogs might call it pyridinium ionic liquid or drop the “1-” prefix. Before making a purchase or planning a reaction, researchers check structures and not just names to be sure they're not accidentally swapping in a methylated cousin or grabbing a product with the wrong counter-ion.
Safety & Operational Standards
Safety discussions ramp up fast with this ionic liquid. Even if it performs better than traditional organic solvents from a volatility point, long-term skin and mucous membrane exposure can cause irritation. Best results come from using sealed systems, chemically resistant gloves, and splash goggles in close quarters. Some protocols require air monitoring for fluorinated dust. Disposal never means dumping it down the drain; incineration under controlled conditions to break the C-F bonds works best. Manufacturers supply emergency spill procedures, which might include neutralizing agents or absorption with inert materials. The growing list of REACH and TSCA rules gives safety officers a clear path.
Application Area
This compound shines in battery and supercapacitor research, acting as an electrolyte with high ionic conductivity and outstanding electrochemical stability. It also steps up in catalysis, where conventional solvents would evaporate or react away. Metal extraction, polymer synthesis, and even specialized analytical chemistry methods draw from its properties. Pharma companies look at it to dissolve stubborn actives or enable rare reactions. In the lab, its solvent power makes it a candidate for green chemistry projects, bypassing VOC emissions that often plague large reactions. As industries turn to specialty chemicals, new application notes keep emerging, especially where high temperatures and strong redox conditions pose problems for traditional solvents or salts.
Research & Development
Every serious chemistry department today has at least one student or postdoc probing the boundaries of what N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide can do. Funding goes to labs that promise cleaner extraction, longer-lasting batteries, or safer catalysis. Technicians lean on high-performance liquid chromatography, GC-MS, and cyclic voltammetry to verify performance. Some work on immobilizing the liquid in polymer matrices, looking for gel electrolytes that could power lightweight electronics. Other groups build computer models of the structure, searching for clues that hint at the next innovation. Industrial research teams check compatibility with engineering plastics or build high-throughput setups for new separations.
Toxicity Research
Scientists keep a close eye on the compound’s toxicity profile. Early animal studies showed oral and dermal LD50s higher than acetonitrile but still above what most safety teams want in a broadly used solvent. The bis(trifluoromethyl sulfonyl)imide anion raised fears about persistent organofluorine breakdown products in the environment, driving additional examination of wastewater and air samples. Modern studies look not only at acute toxicity, but also bioaccumulation and long-term mutagenic potential. Environmental chemists argue for clear waste management rules, since evidence shows the compound resists breakdown in soil and waterways. Getting a grip on real-world impacts means pooling data from industrial and academic settings, then looping what we learn back to chemists and regulators alike.
Future Prospects
Looking ahead, N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide stands at a crossroads. The march toward low-carbon and high-efficiency tech puts pressure on chemists to keep improving ionic liquids’ usability and reduce hazards. Some envision safer, biodegradable analogs that keep the same high-performance features. Electrochemical device designers hope next-generation versions will enhance the life of everything from phones to grid-scale batteries, while waste-management teams watch for tighter rules on disposal. Real momentum gathers around custom blends that target niche needs, giving engineers new power to fine-tune reactions at the bench or on the factory floor. Success, in my view, will depend as much on transparency and safety as on pure technical performance. With careful stewardship, this ionic liquid could carve a place in tomorrow’s cleaner, smarter chemical factories.
Reliable Electrolyte for Batteries and Supercapacitors
My time spent in a university lab watching researchers work with advanced batteries helped me see why chemists pay attention to ionic liquids like N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide (often called [C4Py][NTf2]). Battery engineers face real frustration over liquid electrolytes boiling off, corroding metals, or even starting fires. Unlike the old carbonate solvents, this ionic liquid stands up to heat and sparks with almost extraordinary stubbornness. Tests show it keeps its cool at temperatures that would destroy regular electrolytes, making it ideal for high-power devices like grid storage batteries and electric vehicles. In one project, just swapping in this ionic liquid extended the lifecycle of the battery pack beyond numbers we’d seen before.
Supercapacitor developers also use [C4Py][NTf2] for its larger electrochemical stability window. Supercapacitors crave electrolytes that don’t degrade under stress. With this material, they get better cycle durability, supporting longer use without performance dropping off. Researchers have reported energy densities going up, which brings these energy storage solutions closer to practical everyday use.
Helping Chemists Clean Up Industry
Traditional solvents dump loads of pollution into waterways and air. During my time consulting with green chemistry startups, I saw how the industry needed solvers—chemicals that wouldn’t damage health or the planet. [C4Py][NTf2] resists evaporating into the air and doesn’t burn easily, so it lets chemists pull off key reactions without breathing hazards or fire risks. Many labs rely on it for tasks like extracting proteins or dissolving stubborn polymers, all with less waste and almost no stink.
Pharmaceutical manufacturers grab onto this stuff for tough separations. I noticed how it dissolves organic and inorganic compounds at the same time, making it great for purifying specialty drugs or separating trace ingredients. This versatility means labs can make medicines faster, with less complicated waste handling.
Lubricant for Tough Jobs
Mechanical engineers need lubricants that won’t break down under electric currents or high heat—think robotics, precision gears, or circuit switches. [C4Py][NTf2] keeps its thickness and lubricating power even in demanding environments. Some electric vehicle motors now include ionic liquid-based grease, boosting motor life and reliability. The added bonus: it barely attracts dust or dirt, so cleaning and maintenance get easier.
Safe Conductive Medium for Electroplating
During a tour of a factory plating thin layers of metals onto parts, I watched workers battle with acidic baths and harsh solvents that pitted their tools and seeped into groundwater. Using [C4Py][NTf2] makes those baths less corrosive. The ionic liquid holds metal ions in a stable form, letting manufacturers deposit smooth, even metal coatings for electronics or aerospace parts. Thankfully, spills carry much lower risk, and the finished coatings can hit higher quality marks with less process worry.
Making Sensors Smarter
Engineers designing sensors—those gadgets buried everywhere in modern cars and factories—look for stable materials able to transmit ionic signals cleanly. [C4Py][NTf2] resists electrical breakdown and keeps contaminants out. Its stability means sensors last longer and put out less noise, which matters in medical equipment or pollution monitors where fussy readings can mean life or death.
After seeing the mess left behind by standard industrial chemicals, it’s obvious why these ionic liquids catch the attention of forward-thinking teams. People don’t just chase efficient results—they want solutions that help rather than hurt. This compound offers a toolkit for safer, smarter manufacturing and cleaner batteries, right now—without waiting for a distant future breakthrough.
Safe Storage—It Begins With Temperature and Moisture
Nobody enjoys losing a batch of product to something as simple as the wrong thermostat setting. In my work with both food and chemicals, I’ve seen problems start from a single overlooked label. This product does its best work at a specific temperature, usually cool and dry—heat or humidity creeps in, and trouble isn’t far behind. Mold, clumping, or worse can hit within days. For any team handling bulk ingredients or specialized materials, moisture meters and reliable thermometers make life easier. If you let it slide, cleanup and lost product will eat profits fast.
Keep containers tightly closed and off the bare floor. I use pallets or shelving in every storage room, not just to avoid spills, but also to keep dust and pests at bay. Airflow matters—a stuffy, damp storage closet leads straight to headaches down the line. Some workplaces bring in dehumidifiers during humid seasons, which makes a real difference.
Label Everything for Clarity and Safety
Clear labels do more than spare you a frantic search on a busy morning. Running a shared workspace, I learned early that labels in bold print, ideally color-coded, reduce accidents and prevent mix-ups. It sounds simple, but it’s bad news running into an unmarked drum and playing guessing games. Include expiration dates and handling warnings right on the containers—no one likes surprises in this business.
For hazardous materials, standardized pictograms from bodies like OSHA or GHS cut confusion. I make sure that anyone handling products for the first time knows what those graphics mean. In places with multiple languages, labels cover all bases; it’s not just about compliance, but about respect for every worker on the floor.
Avoiding Cross-Contamination: Common Sense Is Key
From bakeries to biotech labs, I’ve learned that keeping tools and surfaces clean is half the battle. Once, in a kitchen, a colleague used a scoop from one bin to another, and the cross-contamination ruined hours of preparation. One product picking up crumbs or traces of another can bring on customer complaints, recalls, or allergic reactions. A set of dedicated scoops, gloves, and wipes solves this quickly.
Washing hands before and after handling saves more than just time. In industrial work, gloves add another shield, but only if you change them between products. Routine cleaning schedules stop build-ups, especially under shelving or in corners where spills go unnoticed.
Proper Disposal Protects People and the Planet
One mistake I’ve seen too often is emptying leftover product down the drain or leaving it in an open bin. Disposal instructions from the supplier should be clear and followed without shortcuts. In my time at a municipal waste center, I saw first-hand how casual dumping pollutes, sometimes landing businesses in legal trouble. Joining collection programs or working with approved disposal firms prevents costly errors.
Training Powers the Process
Even when processes seem basic, turnover or inexperience can erase hard-won habits overnight. Regular training sessions, including walkthroughs and drills, build confidence. I encourage questions and updates from the staff, since people closest to daily handling spot changes and risks before anyone else. Investing in people beats any shortcut.
Why Purity Matters in the Lab and Industry
Few things create bigger headaches in experimental work than an impure reagent. Anybody who has worked with ionic liquids like N-Butyl Pyridinium Bis(Trifluoromethyl Sulfonyl)Imide (often called [BPY][TFSI]) knows the purity question is not just academic. Even a trace amount of water or leftover starting material can throw off conductivity, solubility, or electrochemical stability. A chemist sees unexpected peaks in NMR or a strange drift in cyclic voltammetry, and immediately suspects impurities. Without clean chemicals, published results wobble, and industrial runs hit snags.
Purity Levels and Analytical Certainty
Labs describe purity in percentages, and for [BPY][TFSI], suppliers often offer 98% or above. Sometimes the bottle says "99%+," and researchers sigh in relief, especially for demanding applications like battery electrolytes or catalysis. At these levels, you get fewer surprises. The rest—usually less than 2%—includes water, trace solvents, and maybe some leftover pyridine. Analytical techniques like HPLC, NMR, Karl Fischer titration, and mass spec help nail down these numbers.
My own grad school work with ionic liquids drove home how tricky purity really is. I once ran a conductivity series thinking the results tracked with viscosity, but later discovered the sample had picked up moisture from the air. That tiny impurity completely scrambled my data. I never forgot to check with Karl Fischer reagent after that. Good suppliers run these checks for every lot, but it never hurts to double-check, especially if precision matters in the application.
The Stakes for Different Applications
Not every process uses the highest purity. Sometimes a little impurity doesn’t break things for basic extraction or routine separations. Trying to build a lithium-ion battery? Anything below 99% can destroy consistency. Impurities can react, degrade the separator, or poison the electrodes, causing a performance drop. For green chemistry work, trace solvents left in a sample can change how easily the ionic liquid dissolves or switches phases.
Some might brush off purity as just another spec sheet detail, but the costs hit fast. Impurities cost hours of troubleshooting, unexpected maintenance on instruments, or wasted materials. In big plants, even small contamination can snowball into shutdowns, off-spec product, or safety hazards.
Fact Checking and Reliable Sourcing
A 2021 Journal of Physical Chemistry Letters article detailed the wide range of “high purity” ionic liquids available commercially. Even within the 98%–99% range, differences pop up between suppliers. Some sell “battery grade” products with tight specs — water content below 50 ppm, halide levels tracked, all checked by certificate of analysis (COA). Always ask for the COA. If a supplier shrugs or gets vague, look elsewhere.
Some labs take extra steps: passing the ionic liquid through activated charcoal, drying under vacuum, or recrystallizing. Those steps eat time and money, but the payoff comes in more trustworthy results and lower failure rates.
Seeking Higher Standards
Industry and research both benefit when expectations for purity push higher. Waste drops. Reliability improves. Good habits—confirming numbers by multiple tests, storing chemicals away from open air—keep projects on track. Chemists who check their reagents and suppliers who back up their labels both steer the field in the right direction. It’s a lesson that goes well beyond ionic liquids.
Meeting Large-Scale Demand
In my experience working with manufacturers, few things slow down a production line more than waiting on small shipments of a needed material. Businesses running large equipment or supplying goods to other industries face a world quite different from consumer markets. Orders come by the pallet or the ton. Delivery schedules matter. Consistent quality matters even more. It’s not just about whether a product can be bought in bulk—it’s about how reliable that supply chain is, and whether you can trust the quality every single time.
Why Bulk Matters
Industrial managers watch budgets carefully. Buying in bulk often helps cut costs, and cutting costs helps companies remain competitive. I’ve seen projects delayed because sourcing managers chased down dozens of suppliers to scrape together enough product. Bulk supply isn’t just a matter of purchasing larger containers. It requires coordination: Can suppliers handle repeat orders? Will packaging protect the product on its way to the factory? Will the next batch deliver the same performance?
Safety can’t be ignored. Splitting up bulk product into smaller containers opens the door for accidents. Take paints, chemicals, or additives—each time a drum is opened and redistributed, the risk of spills, contamination, or exposure rises. In industries like food, pharmaceuticals, or electronics, cleanliness and proper handling are more than afterthoughts. Buying direct from a trusted source cuts down on unnecessary steps and keeps products up to regulatory standards.
The Importance of Traceability
Experience teaches that things do go wrong: a batch comes up short, or fails to meet specs. For a factory manager, having bulk quantities from a reputable vendor tightens up traceability. It’s easier to spot problems, recall product, and fix issues when you know exactly where every drum or bag originated. This helps not just with recalls, but also with routine quality audits.
Checking the Right Boxes
A smart buyer checks more than price and volume. Ask how the supplier stores and ships the product. Find out if their team can handle custom sizes or packaging fit for your operation. I want to see certificates of analysis, full ingredient breakdowns, and confirmation they follow proper safety guidelines. Anything less, and a bargain turns into a waste of time and money.
Challenges and Solutions
Even big distributors stumble. Storms slow down transport, global shortages push up prices, and sometimes container loads arrive damaged. A solution that works well is building relationships with multiple suppliers. Having a backup plan reduces stress when orders fall through. Some companies bring in value-added services, such as supplier-managed inventory, where the supplier monitors usage and keeps stock automatically replenished on site.
Technology helps with these challenges. Real-time inventory systems and sensors keep tabs on usage so orders go out before supplies run low. E-procurement platforms let companies compare supplier ratings and see which ones handle bulk shipping without problems. I’ve talked to managers who swear by these systems for keeping operations smooth year after year.
Moving Forward
Bulk product supply shapes the backbone of many industries. Finding a partner who understands large-scale logistics, follows best practices, and keeps an open line of communication often matters just as much as the product itself. Being proactive, asking tough questions, and investing in reliable systems makes the difference between smooth sailing and one headache after another.
Understanding Everyday Exposure and the Realities of Risk
Most folks never think twice about the chemicals woven into daily routines. Cleaners under the sink, garden treatments, food preservatives—each carries its own safety story. Folks who’ve ever seen a friend wind up with a nasty rash from mixing ammonia and bleach in a well-meaning attempt to deep-clean the bathroom can tell you chemicals demand respect. Toxicity isn’t always about swallowing something bad; sometimes the simple act of breathing in a vapor or letting a drop touch bare skin sets off a chain reaction in the body.
What troubles me is how easy it is to overlook long-term effects. Out of sight becomes out of mind, especially when immediate symptoms don’t appear. Take solvents, for example—some release fumes invisible to the eye, but people using them all day might find headaches, dizziness, or nausea creeping up. Others, like pesticides, can linger in the air or soak into clothing, causing trouble much later. There’s plenty of research showing links between exposure and chronic conditions down the road. The CDC and EPA have pages full of studies connecting chemical mishaps at work or home to problems like liver damage and even cancer.
Learning from Mistakes—Firsthand and Otherwise
Years working in a hardware store stuck with me. I saw everything from cautious shoppers gearing up with goggles and gloves to others tossing strong acids in their carts like they were picking up cereal. One guy I knew landed in the ER after spraying a degreaser in a cramped space without cracking a window. It’s easy to miss caution labels or ignore directions, but ignoring safety steps leads to trouble. Child-resistant caps, warning symbols, and detailed guidance are there for good reason. Seeing the fallout up close always made me appreciate the little details—like labeling leftovers and never putting chemicals into unmarked bottles.
Chemical containers matter, and so does storage. Warm garages increase vapor pressure, leading to leaks. Sealed cabinets keep dangerous products out of reach of kids and pets. Simple steps, but lives have turned on less.
Balancing Convenience and Protection
Gloves, masks, proper ventilation—some roll their eyes and see overkill, but turning safety into a habit trumps handling emergencies. For folks mixing pool chemicals or painters working with acrylics, basic personal protective equipment (PPE) means breathing easier at night. MSDS (Material Safety Data Sheets) and manufacturer notices are goldmines of advice not worth skipping. Taking a few minutes to review this info shields not just yourself, but anyone else sharing the same air or environment.
Doctors and poison control centers urge immediate rinsing and seeking help if skin or eyes meet chemicals, instead of waiting for irritation to set in. Sometimes, an allergic reaction or underlying health factor means even a small exposure tips the body over the edge. Facts show that more than two million poison exposures get logged in the U.S. each year, with household chemicals leading the pack.
Building Better Habits and Spreading the Word
Speaking up in the community shapes attitudes. More teachers weaving chemical safety into lessons makes a difference. Nobody wants to read another tragic news story about accidental poisonings. Families, neighbors, and employers can all play a part by showing safe handling in action, not just making checklists. Reading that label, grabbing gloves, and cracking a window never feel excessive after seeing one accident ruin a loved one’s day or health.
Anyone working with chemicals—from gardeners to folks running a home lab—benefits from knowing the risks and sticking to best practices. Small steps stack up to big safety. As the world leans harder on chemicals in everything from food to new tech, looking out for one another means a lot more than keeping a tidy shelf.