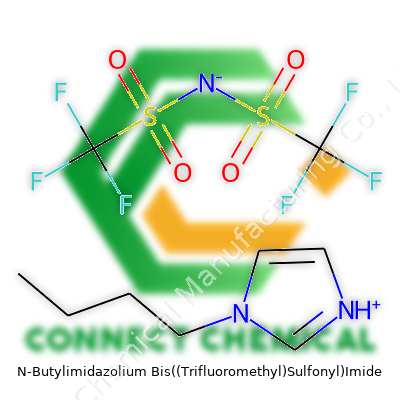
N-Butylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: Roots, Reality, and Beyond
Historical Development
Stories of modern chemistry often lead us to unsung molecules that quietly shape new tools and approaches. N-Butylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, often called [Bmim][NTf2] among those who live and breathe ionic liquids, came as a result of people searching for alternatives to traditional solvents. Scientists recognized limits in water, alcohols, and common organic solvents—flammability, volatility, toxicity, waste. Ionic liquids, especially those built from imidazolium cations paired with bulky, weakly coordinating anions, caught the eye as the late 20th century edged into the new millennium. With [Bmim][NTf2] emerging, chemists saw not just a novel substance but a new class of “green” solutions for old problems, especially as the demand for cleaner, safer, and more efficient industrial processes grew. The timelines here stretch from the late 1980s, with foundational patents and academic publications, through sharp rises in research in the 2000s as companies put real resources behind these alternatives.
Product Overview
This compound stands out due to its ionic nature. It stays liquid at room temperature which, to a chemist working in a bench lab or a plant, makes a world of difference. Its broad liquid range, striking thermal stability, and impressive chemical resistance open the doors to countless applications. Some buy it as a colorless, nearly odorless oil. Some get it as a high-purity lab bottle, ready for research. It gets used as a solvent, an electrolyte, and sometimes even as a template and catalyst. The push toward electrification, battery development, and greener lab practices continues to increase both the demand and respect for this compound.
Physical & Chemical Properties
Handling this substance, experience teaches you that its viscosity—slightly gooier than water but less than syrup—affects mixing and diffusion in experiments. Its density, routinely measured around 1.4 g/cm³, signals the heavy-fluorinated tail. Worries about flammability fade away here because it simply doesn’t catch fire under typical lab conditions, which anyone who’s fought with volatile solvents appreciates. Its chemical stability means that it doesn’t break down or evaporate easily, even under warm or reactive situations, and this resilience both opens possibilities and raises safety discussions. This ionic liquid also brings a broad electrochemical window, so it doesn’t break apart easily during experiments that push voltages higher, which is gold for battery research.
Technical Specifications & Labeling
Those of us who work with chemicals at precise scales rely heavily on clear technical sheets. Bottles of [Bmim][NTf2] show identification marks, batch numbers, and purity figures, usually at or above 99%. Manufacturing sites test every batch for moisture, halide content, and residual starting materials—users in analytics or electrochemistry demand this, since tiny impurities throw off results in sensitive instruments or batteries. Proper labeling also includes GHS hazard icons: the presence of trifluoromethyl groups and sulfonyl imide anions can pose environmental concern, which regulators track from Europe to Asia.
Preparation Method
Synthesizing this liquid usually happens in two well-controlled stages. Chemists begin by producing the N-butylimidazolium halide (often chloride), then swap out the halide for the bis(trifluoromethylsulfonyl)imide anion using a metathesis, or “double displacement” reaction in water. The byproduct, like sodium chloride, can be removed by careful washing and separation. Each cycle brings opportunities for improvement: purer reagents, cheaper methods, lower waste. Real-life practice often introduces a final purification step by vacuum or activated carbon filtration, which techs know saves downstream headaches.
Chemical Reactions & Modifications
In real-world work, [Bmim][NTf2] stands out for its resistance to most acids and bases. It doesn’t degrade during catalysis, nor does it hydrolyze easily, which helps it last across multiple runs of a chemical process—big money for anyone working at scale. The imidazolium ring provides a handle for tailored modifications, allowing new derivatives for specific needs. Some teams tweak the alkyl chain, throwing longer or bulkier groups onto it, while others swap out the NTf2 anion for custom behavior in separation or electrochemistry. These modifications push the frontiers for solubility, conductivity, and even environmental fate, as each functional group changes how the molecule behaves in both lab and natural surroundings.
Synonyms & Product Names
Among chemists and suppliers, the full IUPAC name barely gets used in conversation—it’s a mouthful, hard to write, and harder to remember. You’ll hear “Bmim NTf2”, “1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide”, or sometimes just “NTf2 Ionic Liquid”. Vendors sometimes brand it with product IDs or proprietary names for cataloguing, but as soon as a researcher sees “Bmim” and “NTf2” together, they know exactly what’s in the bottle. Short, standard abbreviations keep things clear across sales orders, shipping manifests, and regulatory reports.
Safety & Operational Standards
People often imagine non-volatile means non-toxic, but plenty of ionic liquids—including [Bmim][NTf2]—carry risks users need to take seriously. Direct skin or eye exposure brings irritation issues; those handling liters for industrial setups use gloves and eye protection, while lab scientists wear lab coats, splash goggles, and reliable gloves. In larger facilities, chemical resistant aprons and full safety showers stand ready. Waste management matters too; you can’t just dump this substance into the drain without risking penalties from environmental authorities. Air quality stays less threatened than with solvents that vaporize quickly, but spills still call for absorbent pads and controlled collection because environmental persistence lingers as a concern.
Application Area
Real utility shows up across more than just one industry. In electrochemistry, researchers swap [Bmim][NTf2] into supercapacitor and lithium battery designs, capitalizing on both high voltage tolerance and thermal stability. The low vapor pressure matters when running processes at sixty, one hundred degrees or more, where classic solvents would evaporate or even ignite. In catalyst and organic synthesis, the liquid stands as both reaction medium and, on occasion, a co-catalyst—offering unique solvation properties that drive better yields or cleaner separation steps compared to old-school solvents. Extraction and separation benefit from the compound’s selective solvency; teams run metal recycling, pharmaceutical separation, or even cellulose processing using these ionic liquids. Some academic labs have begun to look at using [Bmim][NTf2] in analytical chemistry, enhancing detection or separation for challenging analytes.
Research & Development
Momentum in R&D for [Bmim][NTf2] runs strong, with energy storage and waste minimization at the forefront. Production scale-up continues, aiming for lower impurity levels and less expensive manufacturing. Academic groups publish studies focusing on improving ionic conductivity, reducing toxicity, and adapting new, more biodegradable anions. Several projects test customized imidazolium variations to match tougher environmental requirements set by regulatory agencies in the EU and US. Industry consortia collaborate to validate industrial-scale applications, focusing on recycling and recovery to minimize loss and leakage during use.
Toxicity Research
Early optimism about “green chemistry” washed up against reality—ionic doesn’t always mean harmless. [Bmim][NTf2] draws attention for environmental toxicity, particularly toward aquatic organisms and microbiota. Studies highlight limited biodegradability and potential buildup, so disposal guidelines and use restrictions keep getting sharpened. Some animal studies indicate moderate toxicity at high doses; skin contact, ingestion, or inhalation, while less of a risk than common organic solvents, still calls for standard lab safety protocols. Governments watch closely, and chemists keep records religiously now, charting environmental releases, exposures, and containment incidents. This compound serves as a warning beacon: new materials must earn their “green” stripes real with data, not just with marketing.
Future Prospects
Demand for safer, more robust, and more sustainable ionic liquids continues to rise across chemistry and materials science. Battery makers strain to find better electrolytes for the next wave of electric cars, grid storage, and portable gadgets. Recycling and circular chemistry get prioritized, and teams work to develop ways to reuse old ionic liquid stock instead of tossing it out after use. Improved understanding of toxicity pathways pushes development toward more biodegradable ionic liquids. People working day-to-day with these materials want practical changes—cheaper manufacturing, faster purification, safer disposal, reliable supply chains, and easy integration into both academic and industrial pipelines. What happens next hinges on combining solid science, manufacturing scale, and honest acknowledgment of both promise and pitfalls with these complex, fascinating liquids.
An Unusual Yet Practical Ionic Liquid
This chemical name might twist the tongue, but N-Butylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide has turned up in laboratories and industrial setups for good reason. Folks working in research, chemical manufacturing, and green chemistry circles talk about it like it holds a special toolkit for the future. The compound comes as an ionic liquid, which means it stays liquid under reasonable conditions—something that brings flexibility and saves energy during processes that typically demand solvents.
Beyond Ordinary Solvents: A Greener Option
Back in my grad school days, most of our reactions happened in old-school organic solvents—flammable stuff, easy to breathe in by accident, and not great for the lungs. Switching to ionic liquids, especially one like this, cuts down on volatility. Researchers use it to swap out the nasty solvents and get a safer workspace. It's not just about health in the lab: these liquids often break down much less in heat and don’t vaporize at the drop of a hat. That matters for workers, and it matters for the air we share.
Inside the Battery: The Push Toward Better Energy Storage
Battery work has exploded in the last decade. Whether building better electric cars or powering up smartphones, everyone wants better batteries. The electrolyte—the “juice” that moves charge inside—usually consists of watery or flammable materials. This ionic liquid gets picked because it won’t burn, even when things heat up. It handles tough conditions, so engineers use it for lithium batteries and sometimes even for sodium and magnesium ones. The drive to ditch flammable solutions has pointed a lot of funding toward materials like this.
Separating What Matters—Extraction and Purification
Ionic liquids have a knack for pulling out rare-earth metals, precious catalysts, or dyes from waste streams. This one stands out because of its stability and its talent for dissolving both organic and inorganic compounds. Real-world recycling plants and mining operations now turn to these solutions, shifting toward higher yield with less junk left behind. Instead of burning off valuable metals or using harsh acids, companies find a quieter way to recover what counts.
Industrial Synthesis: Cleaner Processes, Cleaner Products
Companies in the chemical sector run reactions that either make medicine or produce performance materials—think plastics or specialty coatings. Every step that leans on safer solvents can lower cost and cut environmental headaches. Using this ionic liquid helps reactions happen faster or at lower temperatures, too. I once watched a colleague switch his entire protocol from a solvent that gave him headaches to this stuff. No smell, fewer disposal issues, and the reaction went just as planned.
Challenges and What Comes Next
Despite all the upsides, cost remains a real headache for widespread adoption. This material doesn’t show up in barrels for pennies. Researchers also spend a lot of energy figuring out how to recycle it well, without losing the benefits that drew them in. The move to bring down prices needs better manufacturing technology and strong demand from big industries. But the chemistry offers hope. Each time someone figures out how to pull off cleaner extractions, quieter batteries, and safer processes, the trade-off gets easier for companies weighing profit versus progress.
Understanding Stability in Real Life
Storing chemicals isn’t just a checklist in a lab. I’ve watched talented technicians waste entire days cleaning up after a bottle started leaking or a little humidity snuck through a cap. Stability isn’t an abstract word. It saves time, budget, and sometimes reputation. Chemicals break down for all sorts of reasons—heat, light, oxygen, water in the air—all of these slowly chip away at purity and strength before you can even use the compound for its real purpose.
Why Conditions Matter So Much
Some compounds react to light. Years ago in grad school, I had a sample turn from white to pale yellow after sitting out by a window over the weekend. That cut its effectiveness down, and no one was happy. Light-sensitive chemistries need brown bottles and dark drawers. Oxidation sneaks in every time someone leaves a cap loose. Water-sensitive compounds don’t last long in regular air, so you learn fast to label things well and use dry boxes or desiccators with fresh packs.
Even small temperature changes make a difference. Walk into any pharmaceutical plant, and you’ll notice the strict temperature logs. Enzyme-based products need cool storage, sometimes at temperatures most home freezers can’t reach. On the other side, volatile liquids or reactive solids require stable room temperatures far from anything that might spark.
Humidity: The Hidden Enemy
Humidity can make powders cake, crystals stick together, and sometimes cause actual decomposition. I once lost a critical run of a moisture-sensitive catalyst because the storage cabinet sat right next to a bathroom. Ever since then, I started keeping sensitive containers with silica gel packets and made sure lids clicked into place. It seems simple, but labs without enough dry storage end up buying stock over and over.
Safety and Labeling Matter in Every Step
Labels can mean the difference between a safe space and an ER trip. Dangerous chemicals don’t always show when they’re starting to break down, but some leak fumes or create peroxides over time. I learned to check expiry dates like I was grocery shopping because old chemicals sometimes go off unexpectedly. Even in professional settings, I've seen junior staff dump containers together, thinking they’re harmless, only for the mixture to bubble or spit.
Regulations aren't just a burden—they keep people safe. OSHA, the EPA, and the European Chemicals Agency set rules based on years of accidents, and the paperwork forces real attention to shelf life and compatible storage partners. You look at what reacts with what, store acids apart from bases, organics away from oxidizers, and keep an updated record of every purchase and disposal.
Repairing Bad Practices
Bad storage habits end up costing real cash. In my early days at a production site, management didn’t budget for a dry room. Within six months, we lost a third of our delicate chemicals to moisture damage, and one batch grew so contaminated with atmospheric carbon dioxide it couldn’t be used. Lesson learned—storage conditions are never just an afterthought for a company or a home experimenter.
Regular audits of storage areas and proper training for anyone handling chemicals change outcomes. Simple steps—using airtight containers, rotating stock, segregating incompatibles, tracking temperatures—pay off. In the end, chemical stability is about protecting investment and safety. Precision here earns trust from everyone, from a researcher at the bench to the end user counting on a safe, reliable product.
What’s the Real Risk?
Dealing with chemicals that sound like tongue-twisters always gives me a pause for thought. N-Butylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, often labeled “ionic liquid,” might not ring a bell outside labs, but inside, folks know it for unique properties. People use it in battery research, electrochemistry, and high-tech fields, so the question often shows up — is it safe?
Many tout the “green” reputation of ionic liquids, including this one. It doesn’t easily evaporate, doesn’t stink, and won’t catch fire like some old-school solvents. That sounds great until you look closer. Just because it doesn't flare up or fill a room with fumes doesn’t mean it keeps you out of trouble.
What Science Says
Touching or inhaling this stuff brings risks. Toxicology data on N-Butylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide still grows, but existing research throws up warnings. Studies show it can cause skin and serious eye irritation. Get it on your hands, you might notice redness or a rash. In the eyes, the pain can last. Accidental inhalation, especially dust or vapor, may irritate mucous membranes or worse, depending on how much you take in.
Long-term health effects remain murky because thorough studies take years. Early evidence from cell and animal tests shows cellular toxicity at higher doses, including possible organ damage. The jury’s out about long-term cancer risk, but no one wants to volunteer as a guinea pig. Chronic exposure, accidental spills, and slack routines raise the stakes for researchers and workers.
Environmental Questions
N-Butylimidazolium compounds break down slowly. When they escape drains or trash, they stick around in soil and water. Some studies point to risks for aquatic life, affecting algae and small freshwater creatures at relatively low levels. The fluorinated part of this molecule sticks and doesn’t let go, lingering for years. Environmental persistence brings up tough choices for disposal and accidents.
Better Handling Practices
After a few decades in chemistry spaces, I learned firsthand that safety rules matter most when they seem boring. Rubber gloves, goggles, and good ventilation should be non-negotiable, even with liquids sold as “safer alternatives.” Preparedness cuts risk further — neutralizing small spills, labeling containers clearly, and knowing where to run if something splashes or spills.
Waste needs careful attention. Ordinary sinks don’t cut it. Labs run these liquids through waste management systems built for industrial toxins. Disposal through licensed channels protects not just workers but water, wildlife, and people living downwind.
Toward Safer Science
N-Butylimidazolium-based ionic liquids deliver new tech, but greener labels don’t erase real hazards. Every time workplace safety slips, exposures rise and so do risks, even if nobody notices right away. Understanding toxic effects, sharing experience, and demanding up-to-date safety sheets for every bottle backs up healthier, smarter research. We all benefit when chemists, students, and workers see beyond a product’s “green” branding and keep safety routines just as tough as for old solvents.
The Basics Everyone Notices
You don’t need a lab coat to spot how some products melt fast on a hot day while others keep their form. Melting point tells you not just how a product reacts to temperature, but how it’ll behave under real-world torment. Take butter and chocolate. Leave both in the sun, and you quickly see one puddles faster than the other. For manufacturers, this property signals how a product travels, gets stored, or stands up during shipping in stifling heat or biting cold.
Pharmaceuticals and food industries keep a close eye on melting point for another reason—purity. If a tablet starts turning mushy at room temperature, shelf life goes out the window. Synthetic chemists trust that the sharpness of the melting point hints at product cleanliness. Compounds loaded with impurities tend to melt in a messy, uneven fashion.
Solubility Shapes Usefulness
Solubility doesn’t just show off in beakers; it rules the real-world usefulness of products. Think back to making a morning cup of instant coffee. If the powder clumps and floats, it annoys everyone. Water solubility matters in everything from pain relievers to cleaning supplies. High solubility usually makes mixing into drinks, solutions, or even creams much easier. It’s a deal breaker for drug companies—medicine that dissolves quickly gets absorbed effectively.
Not every product needs to dissolve. Water-resistant materials lean the other way. I once ruined a favorite jacket by assuming it could handle a sudden downpour. Some fabric coatings and plastics stay outside the reach of water, thanks to stubborn solubility. Understanding how a substance interacts with water, oils, or alcohols influences where you’ll find it in stores or medicine cabinets.
Big Choices Come Down to Gravity, Not Guesswork
These properties might sound technical, but they drive big decisions. Everyday folks don’t always get to see how much thought companies put into making products survive at home, at work, or in unpredictable weather. If a cleaning spray refuses to dissolve, or if a candy bar liquefies before lunch, the problem lands back at the blueprint stage—someone guessed wrong about physical behavior.
I’ve watched coworkers in labs run three-day checks just to chart how fast something melts or how easily it dissolves. There’s good reason for all this. Melting point and solubility put a stop to wasted resources, unhappy customers, or even unsafe conditions. Once I saw a production team scramble to fix an issue after a snowstorm revealed their product wasn’t as cold-resistant as hoped. They took samples, tested again, and tweaked formulas until the issue vanished.
Fixing the Trouble Spots
Problems often come from bad predictions or skipped tests. Labs need tools like proper heating setups, clean glassware, and detailed procedures. Some teams use additives or tweak chemical structures to hit the sweet spot for melting point or solubility. For example, adding different salts or switching out a single part of a molecule can transform how a compound behaves.
Creative problem solving sometimes makes all the difference. If a topical cream feels gritty or dries out, a simple switch in the formula or finding a more soluble ingredient can solve it. Manufacturers need feedback from both science teams and people using the product. Open communication keeps the next batch from repeating the old mistakes.
Why Buyers Should Care
Physical properties aren’t just numbers in a book—they decide how easily life’s little rituals flow. Selecting the right cooking fat, picking pills that dissolve faster, or choosing weatherproof gear comes down to knowing how these materials handle stress, moisture, or heat. My own trial-and-error in the kitchen, lab, and at home keeps reminding me how these overlooked details decide what ends up in our carts and hands every day.
Understanding What’s at Stake
N-Butylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, abbreviated as [BMIM][NTf2], shows up in labs that chase cleaner chemical reactions. This ionic liquid finds a home in electrosynthesis, advanced batteries, and separation technologies. People feel drawn to it because of its stability and non-volatile nature, but that same endurance complicates disposal. It doesn’t simply break down, so tossing leftovers down the drain or adding them to regular waste introduces real risk.
Energetic Chemicals Demand Responsibility
There’s reassurance in studies showing [BMIM][NTf2] slips through the environment less easily than traditional solvents. Its low vapor pressure helps keep it out of the air, but research from the last few years points to stubborn behavior in water and soil. A 2022 study in Chemosphere uncovered traces persisting up to weeks in aquatic settings. Once released, it doesn’t just vanish; there’s a window for bioaccumulation and harm to aquatic organisms. I’ve watched respected peers puzzle over slight shifts in ecotoxicity data and accept that neglecting a few milliliters here or there can leave a mark for seasons to come.
The Human Factor in Safe Disposal
Many chemicals reward strict boundaries, but ionic liquids like this one ask for undivided attention. If poured down drains, [BMIM][NTf2] could reach waterways that feed into larger rivers or city reservoirs. No wastewater treatment system today fully clears these resilient molecules. Any slip in protocol multiplies risk for fish, microbes, and possibly people relying on that water downstream. Working in academic or industrial labs, I’ve seen waste containers marked clearly—one for halogenated solvents, another for acids, and still another for specialty waste like ionic liquids. Training new staff means showing, not telling: secure those lids, double-check the tags, never assume trace residues are harmless.
Practical Steps Toward Safer Outcomes
Like many advanced materials, [BMIM][NTf2] resists neat solutions. Burning it may release noxious fluorinated gases, and simple landfill burial risks leaching. Incineration under controlled, high-temperature conditions—preferably above 1000°C—offers the surest neutralization. This approach costs money and depends on facilities designed to scrub the resulting gases, but for hazardous organic salts, it removes blind spots in responsibility.
Separation and recovery from used reaction mixtures can cut total waste. In some projects, teams distill off water or acids, leaving a residue of ionic liquid that gets reused for another round. Frequent recycling dulls the shock of fresh disposal and lowers the total risk to the environment. Still, impurities accumulate, forcing eventual discard under secured conditions.
Solutions Rely on Real People
Lab personnel, managers, and environment officers need to shape clear rules, reinforced with regular reminders. Documented procedures, inventory logs, and cross-checks tighten the routine. Regulatory agencies like the EPA and their European counterparts urge organizations to submit disposal profiles for new substances, and modern labs know that detailed record-keeping builds trust with regulators and the local community. No container should ever sit in a forgotten corner, nor should anyone hope a mop-up job means a chemical simply disappears.
Community outreach—posters in break rooms, required annual safety refreshers, and open-door policies for error reporting—bakes accountability into the culture. Mistakes shrink when workers feel supported instead of blamed. My years in shared research spaces taught me that proper disposal doesn’t happen by accident. It grows out of teams with skin in the game, motivated by genuine care for each other’s well-being and the world around them.