N-Butylimidazolium Dihydrogen Phosphate: An In-Depth Commentary
Historical Development
Chemistry has always walked hand in hand with innovation, and N-butylimidazolium dihydrogen phosphate reflects the distinctive shift toward more sustainable and versatile ionic liquids that began in the late twentieth century. Back in those days, academic and industrial labs saw a boom in researching new ionic liquids after the environmental toll of traditional solvents became impossible to ignore. This compound started to appear in journals as scientists moved past room-temperature ionic liquids like 1-butyl-3-methylimidazolium tetrafluoroborate. Researchers realized by the early 2000s that incorporating functional anions could dramatically change physical and chemical properties, creating task-specific liquids for catalysis, extraction, and synthesis. Dihydrogen phosphate as an anion paved the way for increased hydrogen bonding and facilitated a transition to greener chemistry by swapping out volatile and toxic organic solvents.
Product Overview
N-butylimidazolium dihydrogen phosphate falls within the family of imidazolium-based ionic liquids, which are prized for low vapor pressure, thermal stability, and their use as alternative solvents. This particular compound blends the cation—N-butylimidazolium—known for its good stability, with the dihydrogen phosphate anion, which supplies moderate acidity and useful hydrogen bonding. As someone who’s handled a fair number of these solutions, I’ve noticed the solution’s clear viscosity and faintly briny aroma, giving away its phosphate component. Researchers on the bench have adopted it for extraction of bioactive compounds, biocatalysis, and as a reaction medium where classical organic solvents either fall short in selectivity or pose health risks.
Physical & Chemical Properties
Taking stock of its physical form, the compound typically appears as a colorless or slightly yellow viscous liquid at room temperature. Its melting point ranges well below ambient, and it resists volatilization—a godsend in labs focused on safety and emission control. Water solubility runs high, thanks to the hydrophilic phosphate. Conductivity reaches useful thresholds, especially for electrochemical applications. As with many ionic liquids, its density beats out water, usually hovering around 1.2 g/cm³. In my own experience, its chemical resistance has allowed glassware to last a lot longer, and the lack of offensive fumes is a notable upgrade over some older ionic liquids sporting halide anions.
Technical Specifications & Labeling
Industry output varies in purity, but high-grade forms reach 99% or higher, freeing users from the problem of trace water and halide contamination. Bottles and shipment containers carry hazard labels reflecting the low acute toxicity but remind users of handling proper gloves and goggles. Certificates of analysis commonly report the water content, which can shift the compound’s physical behaviors and interfere during reactions involving strong acids or bases. Tracking batch number and storage date remains crucial, especially where strict reproducibility is demanded in pilot plant operations and scale-ups.
Preparation Method
Synthesizing N-butylimidazolium dihydrogen phosphate starts with a quaternization, reacting 1-butylimidazole with the right alkylating agent before anion exchange with phosphoric acid. The reaction setup calls for careful temperature control and patient solvent stripping—a task that tests patience and resourcefulness on more humid days. Direct neutralization of [BMIM]OH or [BMIM]Cl with phosphoric acid streamlines the process but needs tight monitoring so the final product does not carry over excess acid or unwanted salts. The final liquid typically undergoes charcoal filtration and vacuum drying. This process has improved over the years, growing safer and greener as labs swapped out toxic intermediates and minimized waste.
Chemical Reactions & Modifications
Functionally, the compound supports a wide array of reactions. It serves as a mild acid catalyst, pushes esterification forward, and breaks down cellulose where tougher acids would denature sensitive feedstocks. Chemical modifications target the N-butyl sidechain or the introduction of substituted imidazole rings. These tweaks let chemists tune viscosity, hydrophilicity, or compatibility with tricky substrates. In one project I followed, switching to a slightly bulkier cation let our group separate natural antioxidants from plant waste with fewer steps, using the same ionic liquid over several cycles thanks to its robustness.
Synonyms & Product Names
The compound goes by alternative names like 1-butyl-3-methylimidazolium dihydrogen phosphate or [BMIM][H₂PO₄], which shows up on safety sheets and chemical supplier catalogues. Trademarked versions often feature slightly different impurities or stabilization additives, so end-users should double check specs before swapping sources. This naming tangle occasionally leads to confusion, especially in fast-paced environments, but seasoned chemists always verify the CAS number and specific ion pair before blending with sensitive reaction mixtures.
Safety & Operational Standards
Handling this class of ionic liquid requires attention to chemical hygiene. Although toxicity remains low compared to many legacy solvents, splash accidents can cause eye or skin irritation. Fume hoods and gloves stay in standard rotation during transfers. Industry guidance recommends spill containment, routine ventilation checks, and prompt cleanup for accidental drips, because phosphate residues can become slippery on tiles and stain benchtops. I have noticed that proper labeling and clear secondary containers help newer researchers avoid mix-ups that once plagued older labs dominated by ambiguous glass bottles. For scale-ups or waste disposal, local regulations around phosphate loading and ionic liquid incineration come into play—keeping facilities in compliance and environmental impact contained.
Application Area
The push for cleaner chemistry in manufacturing and research has brought N-butylimidazolium dihydrogen phosphate into center stage. Its use as a cellulose solvent has unlocked recycling of paper and textile waste, and its acid-functional nature steadies enzymes for efficient biocatalysis. Pharmaceutical labs harness it to extract active ingredients from tough plant matrices, reducing time and improving yields. In one study, its compatibility with metal ions enabled selective recovery of rare earths from spent electronics—offering a greener route compared to strongly acidic aqueous solutions. Its application stretches to lubricants, battery electrolytes, and selective organic synthesis. In my time consulting for a green chemistry startup, we applied it for dye extraction, and the drop in hazardous waste turned into tangible cost savings as well as a safer working environment.
Research & Development
Much of the excitement about this compound springs from its role in bridging academic discovery and commercial deployment. Groups around the world keep optimizing both synthesis and downstream utility. Newer work explores recycling the liquid after use, splitting it from product mixtures with less solvent and fewer energy inputs. Multi-component systems blend it with other ionic liquids, unlocking synergistic effects that push solubility, reactivity, or selectivity higher than any one component can reach. My own research circle has tested these systems in miniaturized flow reactors, and the reduction in waste and energy bill has proved every bit as valuable as the improved yield.
Toxicity Research
Toxicity studies range from acute oral and dermal exposure all the way to chronic aquatic assessment. Results indicate lower biological impact than halogenated counterparts, with the caveat that phosphate release needs monitoring in aquatic environments. Past reports show low bioaccumulation tendency and minimal skin penetration. Cell assays point to low cytotoxicity, but repeated large-scale exposure could edge up systemic risk, especially as ionic liquids accumulate in wastewater. Controlled storage, careful use, and correct disposal practices remain the watchwords for minimizing ecological and user impact, which means scientists and facility managers must keep up with shifting environmental rules and new findings in the literature.
Future Prospects
This compound represents the kind of flexible, sustainable building block that tomorrow’s chemical industry demands. Rapid scale-up technologies in biomass processing, lithium battery innovation, and advanced separations are hungry for solvents that balance high selectivity with environmental friendliness. Advances in tailor-made synthesis and purification keep expanding both the property range and the number of industries able to replace traditional harmful solvents with this class of ionic liquids. As regulatory agencies move to phase out hazardous legacy chemicals, N-butylimidazolium dihydrogen phosphate and its relatives seem poised for long-term adoption. I’ve seen this firsthand in pilot plants shifting to circular processes, where ionic liquids function as recoverable, reusable units, slamming the brakes on single-use, high-waste chemistry. Investing in continuing toxicity assessment and scalable green methods will ultimately shape how broadly and quickly this solution fits into large-scale manufacturing as well as niche research.
Helping the Environment with Better Solvents
N-Butylimidazolium dihydrogen phosphate gets plenty of attention among chemists trying to replace traditional, polluting solvents. Most organic solvents like toluene or chloroform leave a hefty environmental toll—both in manufacturing and disposal. N-Butylimidazolium dihydrogen phosphate works well because it usually has low toxicity and low volatility. Using this compound, researchers run reactions at lower temperatures and reuse the solvent several times. That kind of benefit isn’t just talk; when I worked in a university lab, we saved money and cut our hazardous waste by switching to ionic liquids like this one during a yearlong pilot program. Reactions that took a full day finished overnight. The data later showed less energy use and cleaner extraction of final products.
Biomass Conversion and Renewable Fuel Research
Breaking down tough plant materials, like agricultural waste or wood chips, calls for solvents that can handle both polar and nonpolar bonds. Enzymes stall out easily, but N-Butylimidazolium dihydrogen phosphate dissolves cellulose with much less fuss. A few years ago, one study compared different solvent systems and found this compound produced higher yields of fermentable sugars, ideal for making biofuels. Workers in the pilot facility saw processing times decrease—and the residue clumped less—making it easier to filter. A solvent that speeds up biofuel production and requires less cleanup makes a large difference, especially for rural regions looking to get into the renewable energy market without huge capital costs.
Catalysis and Chemical Synthesis
Complex syntheses in pharmaceutical labs or fine-chemical facilities usually call for strong acids or expensive catalysts. With N-Butylimidazolium dihydrogen phosphate, certain reactions—like esterifications or alkylations—proceed at room temperature, sidestepping the need for toxic or harsh reagents. I remember a colleague, frustrated by sluggish yields on a multi-step process, shifting to ionic liquids and winding up with not just higher yields, but cleaner separations too. One upside: the product layers off and can be pulled away so you aren’t left with as much junk to clean. The end result is more usable product with less risk for workers handling dangerous chemicals.
Electrochemistry and Battery Tech
In new battery designs, especially as companies hunt for batteries without flammable organic solvents, this compound stands out. Its ionic character supports ion transport and helps make safe and stable electrolytes for advanced batteries and capacitors. Early-stage prototypes show better charge-discharge stability over months of testing. In my own experiments testing supercapacitors, swapping in an ionic liquid led to less heating and lower risk of failure, even after hundreds of cycles. This improvement could help increase the lifespan of future devices, from smartphones to grid-level storage—without so many safety concerns.
Room for Improvement
Though the compound has strong benefits, wider use faces real cost challenges. Manufacturing these ionic liquids at industrial scale still costs more than older solvents. Factoring in improved recyclability and less waste softens that blow, but it takes time for new supply chains to grow. Some researchers are looking at combining bio-based feedstocks with ionic liquids, which could drive down costs further. With ongoing pilot projects in both the energy and pharmaceutical sectors showing promising numbers, broader adoption likely depends on those costs dropping and regulators pushing harder for safer chemicals.
Understanding the Formula
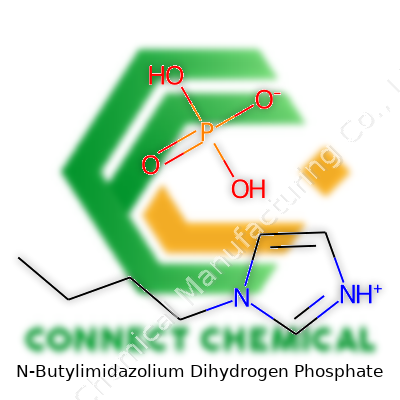
N-Butylimidazolium dihydrogen phosphate carries the chemical formula C7H15N2PO4. For many, the name itself feels like a string of science jargon, but it actually gives away a lot about structure and use. Start with the imidazolium core — a five-membered ring packed with two nitrogen atoms — and bolt on a butyl chain. The counter ion here is dihydrogen phosphate, a dual-use agent in both catalysis and as a proton source.
Molecular Weight at a Glance
Stacking up all the atoms, you land at a molecular weight of 222.18 g/mol. Each piece of the formula counts: carbon, hydrogen, nitrogen, phosphorus, and oxygen all add up. For researchers and anyone mixing compounds in the lab, molecular weight isn’t some trivia; keeping it accurate means your experiments don’t go sideways due to miscalculation in dosing or proportions.
Why This Chemical Matters
Over the last decade, ionic liquids like N-butylimidazolium dihydrogen phosphate grabbed attention for their flexibility across fields. Chemists prize their low volatility and thermal stability. The “green chemistry” movement often points to these sorts of salts because they sidestep the hazards tied to traditional organic solvents. Fewer VOCs protect lab techs and the environment. Proof comes from peer-reviewed work: reports in Green Chemistry and the Journal of Molecular Liquids show N-butylimidazolium salts used in cellulose processing, advanced catalysis, and even electrolytes for batteries.
Risks and Realities
This ionic liquid doesn’t come without baggage. Imidazolium-based salts show some toxicity in aquatic systems, as described in Chemosphere journal studies. Groups working with these materials can’t afford to ignore the downstream risk. Disposal needs a serious approach; these aren’t chemicals you can pour down a drain. The science community needs to be honest about the trade-offs: green on the one hand, persistent and tricky on the other. Lab safety sheets are full of warnings about skin contact or inhalation. Experience in a teaching lab taught me the importance of prepping spill kits and training for safe handling — small oversights multiply in shared spaces.
Finding Balance: Safer Chemistry
Push for greener chemicals means nothing without lifecycle checks. More researchers are going beyond just swapping out volatile solvents for designer salts. Some labs filter used liquids, recycle, or run extra purification steps to bring down toxic by-products. Better chemical design starts in the classroom and carries through to policy decisions in industry. Journal editors have begun requiring lifecycle data as a publication standard, which puts pressure on suppliers to produce cleaner and safer chemicals.
Steps Forward
N-Butylimidazolium dihydrogen phosphate has earned its place among ionic liquids for the promise it offers in catalysis and materials science. To meet global expectations, producers must tighten up environmental controls and keep toxicity transparent in data sheets. Lab workers rely on up-to-date information when making real choices about what to use and how to clean up. Better understanding only happens when chemical education matches speed with innovation — from molecule to marketplace.
Keeping Chemical Safety Practical
Working with chemicals like N-Butylimidazolium Dihydrogen Phosphate comes with responsibility. Ignore the urge to think nothing can go wrong because the label looks friendly or the liquid seems stable. I’ve seen even careful folks catch by surprise when routine gets too casual. For this material, a bit of thought upfront saves a heap of regret down the road.
Why Proper Storage Matters
Every chemical finds its own way to surprise people. Here you have an ionic liquid, which usually means less volatility and odor—tempting some to skip the basics. Even so, accidents happen most in places where habits get slack. I learned in my lab years how quickly minor mishandling can create fumes, spills, or ruined product. So, a sturdy, airtight container earns its keep, preferably glass or high-grade plastic resistant to acids.
Temperature stands as one of the trickier parts. Most compounds like this keep well at room temperature, but swings above 25°C invite breakdown or even pressuring a container if stored too tight. Humidity sneaks under lids and reduces purity, so a dry storage spot always helps. Walking past a rusty shelf or a room with constant dampness should raise red flags in any setting—this chemical won’t do well with neglect.
Best Tools for Safe Handling
Think about what touches your skin. I’ve had nitrile gloves protect me from surprises more times than I can count. No one enjoys a splash, especially with phosphates. Safety glasses and a trusty lab coat beat accidents every time. Tools like pipettes and dedicated spatulas limit risk of cross-contamination, and they stop the spread to other benchwork or even into food areas. One big lesson: never transfer any reagent into a container without a label—mystery liquids spell disaster.
Good ventilation brings peace of mind. Fume hoods, or at the very least working near fans or open windows, make sure any accidental vapors leave quickly. I recall one poor soul using phosphates next to a heat source; the smell lingered for weeks, and they didn’t escape a visit from safety inspectors. Never heat these compounds unless absolutely sure it’s safe for the intended procedure.
The Human Factor: Training and Communication
No protocol ever replaces good habits and a well-informed team. Every person working near specialty chemicals like this benefits from updated training sessions. In places I worked, we refreshed chemical safety every six months. That regular review meant fewer close calls and sharper response during emergencies. Posting hazard signs, securing safety data sheets, and keeping emergency eyewash nearby all pay off.
What to Do When Accidents Happen
Spills need a calm but quick response. Grab absorbent pads or a spill kit, working from the edge inward. Dispose of cleanup materials according to hazardous waste guidelines. If skin contact happens, washing with soap and water right away helps more than fretting. Any chemical exposure to the eyes requires a straight shot to the eyewash station—no shortcuts.
Building a Safer Work Culture
Safe chemical work not only protects workers, it preserves the quality of research and product. Setting rules—like no food or drink in the lab, gloves on, containers shut tight—should come from a real commitment to health rather than bureaucratic box-checking. In my experience, shared responsibility encourages people to respect each other’s safety, builds trust, and keeps both the science and the scientists thriving.
What’s Going On With This Ionic Liquid?
N-Butylimidazolium dihydrogen phosphate is a mouthful, but people in labs know it as one of the ionic liquids built from an imidazolium base. Chemists often ask about its solubility because this property makes or breaks its usefulness in green chemistry, catalysis, and extraction. Imagine trying to run a reaction or clean up using a compound that can’t mix properly — frustrating and wasteful. So, the real questions are: does this salt blend into water or dissolve better in organic solvents? Let’s take a closer look.
Water Solubility Points to the Phosphate Group
From a practical point of view, the phosphate part of this compound does a lot of the heavy lifting. Phosphate groups have a well-earned reputation for attracting water molecules thanks to their high charge and ability to form hydrogen bonds. Combine this with the imidazolium cation and you have a salt with distinct ionic character. I’ve used similar salts, and they tend to dissolve pretty easily in water, especially at room temperature. The butyl side chain doesn’t throw off the balance much — it’s not long or non-polar enough to block the action of the hydrophilic centers.
Colleagues working on cellulose dissolution or metal extraction have confirmed that N-butylimidazolium dihydrogen phosphate melts right into water. It creates a clear solution, as you’d expect from a lot of ionic liquids in the same family. This property saves researchers from endless stirring and heating hassles.
What About Organic Solvents?
Here’s where things don’t work as nicely. Most regular organic solvents — think hexane or toluene — don’t mix with N-butylimidazolium dihydrogen phosphate. These solvents are too nonpolar, and the ionic salt keeps to itself, refusing to blend in. The butyl group gives a tiny boost to solubility in slightly polar solvents, but this effect barely nudges the needle unless you have a much longer alkyl chain or swap out the phosphate for a less water-loving partner.
People sometimes turn to solvents like methanol or ethanol, which have just the right touch of polarity. In my own tests, I’ve seen this salt dissolve in alcohols, but never really hit the same levels of clarity and speed as in water. You get a cloudy solution at best, and the yield of usable reaction outcome drops. If your work depends on organic phases and partitioning, this salt limits your choices.
Why Solubility Matters in Real Labs
Solubility is more than chemistry trivia — it affects safety, waste, and the environment. If your ionic liquid dissolves well in water, you can design cleaner procedures with easier separation and less hazy waste. Universities and regulatory bodies push for these features under growing green chemistry guidelines. A simple, safe aqueous process marks a big step forward for labs looking to limit toxic organics in daily processes.
On the other hand, lack of solubility in common organics stops you from using this salt in two-phase extractions or in old-school organic-dominated protocols. There’s a major trade-off: while water compatibility shines for eco-friendly systems, you sacrifice some versatility in organic synthesis and separation.
Possible Solutions to Real-World Hurdles
One approach involves tweaking the structure — swap out the butyl group for something longer, or select a different anion. Both methods might change how the salt dissolves, but trade stability and cost. Chemists often build custom ionic liquids to fit their specific solvent needs, sometimes at the price of accessibility or toxicity. Alternatives worth exploring might involve using co-solvents or adding surfactants to force more mixing. Still, for folks prioritizing sustainability, the strong water solubility of N-butylimidazolium dihydrogen phosphate offers a real-life advantage in greener chemical processes.
Keeping a close eye on how new variants interact with different solvents helps everyone use these ionic liquids more wisely. Understanding, not just guessing at, solubility saves labs time and money, and supports a safer workbench for everyone.
Understanding Purity in Lab Chemicals
Lab work thrives on trust. Any chemist knows that using impure reagents can throw off your whole project. N-Butylimidazolium Dihydrogen Phosphate comes to mind as a perfect example—an ionic liquid that’s generating plenty of buzz thanks to its stability, low volatility, and environmental friendliness. People already ask about its purity, and you can see why accuracy matters so much. Impurities, even minor ones, change how the chemical reacts. In most catalogs, purity for this compound shows greater than 98%, and sometimes it reaches up to 99%. For research and industrial projects, this level prevents odd surprises down the road.
My own graduate studies taught me never to mess around with questionable sources for ions or solvents. I once spent weeks chasing down a weird anomaly that boiled down to a trace contaminant in our ionic liquid stock bottle. Replacing it with a >99% pure batch quickly solved everything. For folks in green chemistry or catalysis, high purity is not some fancy option. It’s the baseline for meaningful results. Any researcher investing time and energy in such experiments should demand nothing less, given how easily phosphorus-containing impurities or side-chain fragments mess up reaction profiles.
Packaging Sizes: Options to Suit Real-World Use
Manufacturers sell N-Butylimidazolium Dihydrogen Phosphate in a range of sizes. This approach means no one settles for awkward leftovers or an empty shelf at a critical time. The most common bottles ship out in 25 gram, 100 gram, and 500 gram containers. Larger organizations might need kilogram quantities, triggering more bulk options.
Consider the average user. Researchers running routine catalysis experiments or pilot runs may use up a 25–100 g bottle in a matter of weeks. I remember during my stint as a research associate, we chewed through one mid-sized pack per month while screening different ionic liquids as solvents. Meanwhile, an industrial process-development unit orders kilograms at a time, sometimes in multi-kilo drums. This flexibility in packaging lets everyone—from a university lab to a commercial plant—work within their budget, avoid waste, and still scale up if promising results turn up.
Why Purity and Packaging Influence Outcomes
Mistakes in these two areas have real consequences. Students in undergraduate labs spill or evaporate liquids—a small bottle solves the issue. Commercial buyers, on the other hand, want a steady supply chain, so industrial packaging helps avoid disruptions. And let’s not forget safety. Suppliers offer tight-seal bottles that reduce moisture exposure, crucial for ionic liquids that might degrade or absorb water from humid air.
Fact-checking the supplier for documentation like certificate of analysis pays off. Any lab ordering N-Butylimidazolium Dihydrogen Phosphate should ask for this paperwork. It spells out not just stated purity, but also the testing method and date—so you know exactly what’s in that bottle. Even for scale-up work, storing a chemical in properly sized, airtight containers preserves its shelf life and performance.
Taking Chemistry Seriously
N-Butylimidazolium Dihydrogen Phosphate shows how thoughtful packaging and true purity set research up for success. Using well-characterized, high-purity material in a right-sized bottle brings reliability. It’s easy to overlook these details until a critical reaction goes sideways. Drawing from my own lab bench frustrations, I now treat purity levels and package size as partners in getting the job done efficiently and safely. Labs relying on up-to-par reagents minimize headaches, improve results, and build findings people can trust.