N-Butylimidazolium Hydrogen Sulfate: A Straightforward Look at a Modern Chemical
Historical Development
N-Butylimidazolium hydrogen sulfate popped onto chemists’ radar in the early 2000s. Before that, most labs kept their eyes on traditional solvents and reagents, often volatile and toxic, leaving people searching for safer, more effective replacements. Ionic liquids began stepping up as the green chemistry movement grew, and researchers noticed the imidazolium family could provide strong alternatives to flammable, odorous solvents. I spent my grad school years combing through patent filings and academic journals where N-butylimidazolium compounds started carving out a space—the hydrogen sulfate variant drew interest for being less corrosive, relatively less toxic, and frankly, easier to handle than the harsh mineral acids everyone used to store in glass bottles locked deep inside fume hoods.
Product Overview
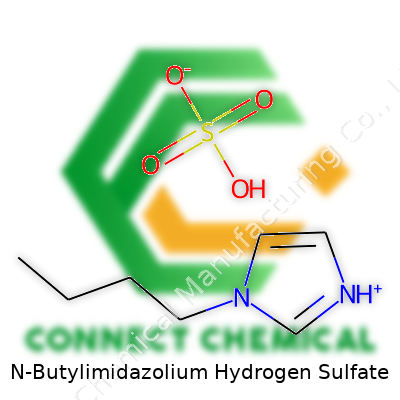
N-Butylimidazolium hydrogen sulfate, known by names like [C4im][HSO4], is one of those colorless to pale yellow liquids that rarely shows up in flashy advertisements but makes its mark in dozens of labs and plants. Its low volatility makes it a favorite where fumes and flammability would otherwise slow a project to a crawl. Unlike the older, more aggressive acids and high-boiling solvents, this compound fits into the growing camp of ionic liquids—liquids made entirely of ions, built for demanding yet sensitive jobs in organic synthesis, catalysis, and extraction work.
Physical & Chemical Properties
I spent days working with these “designer solvents” and grew to appreciate N-butylimidazolium hydrogen sulfate for its consistency. It doesn’t evaporate at room temperature, and its viscosity feels more like syrup than water, making spills much easier to contain. Its melting point usually falls below 0°C, so it rarely crystallizes in typical lab conditions. This property keeps reactions running, especially where temperature swings could freeze other solvents solid. Stable up to around 250°C, it presses on through harsh conditions—something every industrial user quietly wishes for. Its slightly acidic nature gives it mild catalytic behavior, and I often saw it used to replace corrosive mineral acids without sacrificing reaction speed or selectivity.
Technical Specifications & Labeling
Trusted suppliers label N-butylimidazolium hydrogen sulfate by both its empirical formula C7H14N2SO4 and its structure: one N-butylimidazolium cation paired with a hydrogen sulfate anion. Safety Data Sheets tend to break down water content, halide purity, and color parameters. Most bottles contain at least 99% purity, with water content kept as low as possible to limit hydrolysis in sensitive syntheses. If you’re buying it for regulated work, UN and CAS identification stays on all labels for proper tracking in both shipping and disposal processes.
Preparation Method
Synthesis of N-butylimidazolium hydrogen sulfate rarely strays from a two-step protocol. First, you alkylate imidazole with butyl chloride or bromide in the presence of a base, usually in a solvent-free or solvent-light setup to keep things green and efficient. Imidazole is cheap and relatively safe and, when paired with butyl halide, yields the desired butylimidazolium halide. After isolation and purification, this salt meets a stoichiometric amount of sulfuric acid—often dropwise and at low temperature. The exothermic reaction swaps the halide for hydrogen sulfate, and the product separates as a viscous, pale liquid. I’ve seen tweaks, like microwave reactors or sonochemical methods, crank up yields while reducing reaction times. These newer approaches align with the ongoing shift away from waste-generating batch chemistry.
Chemical Reactions & Modifications
N-butylimidazolium hydrogen sulfate frequently acts both as a solvent and as a catalyst, particularly in acid-catalyzed reactions such as esterifications, condensations, and organic rearrangements. From my own benchwork, I know the value of cutting out a hazardous acid by using an ionic liquid that drives the reaction and simplifies separation. Modifications often involve changing the length of the alkyl side chain or swapping in different ionic partners to tune properties like melting point, viscosity, and acidity. Even minor substitutions have pronounced effects, which researchers exploit for creating task-specific media—often for metal extraction, cellulose processing, or even pharmaceutical preparation.
Synonyms & Product Names
You won’t always see the mouthful “N-butylimidazolium hydrogen sulfate” on a bottle. Labs and product catalogs bounce between [BMIM][HSO4], 1-butyl-3-methylimidazolium hydrogen sulfate, or simply [C4im][HSO4]. Some companies create proprietary blends under trade names, but the main identifiers boil down to the core imidazolium ring, the four-carbon butyl chain, and the hydrogen sulfate anion. A smart buyer cross-references CAS (often 262297-13-2) to avoid surprises.
Safety & Operational Standards
Those who worked with older, more volatile solvents know that handling improvements matter a lot. N-butylimidazolium hydrogen sulfate doesn’t catch fire easily, doesn’t evaporate quickly, and rarely emits dangerous fumes at room temperature. Still, it acts as a mild irritant, especially for skin and eyes, and gloves and goggles remain non-negotiable. From my years on the safety committee, I saw fewer spill response incidents with ionic liquids, probably because their low volatility cuts down on accidental inhalation and environmental contamination. Disposal, though less fraught than with strong mineral acids, still demands suitable containment—no pouring down the drain, not even in tiny amounts. Regulations continue to evolve as researchers map environmental effects and long-term breakdown products.
Application Area
Chemists working in synthesis, extraction, and catalysis often rely on N-butylimidazolium hydrogen sulfate. Its knack for dissolving organic and some inorganic compounds opens doors for biphasic reactions. I watched colleagues harness it to drive Fischer esterifications at room temperature or extract bioactive compounds from stubborn plant matrices. Large-scale producers use it for clean-up processes, recycling metals from spent catalysts, and transforming biomass into fuels and materials. I’ve even seen it enter research on battery electrolytes thanks to its thermal stability. Environmental engineers look toward it as a less-toxic alternative for certain industrial processes, hoping for lower ecological risk and easier containment compared to traditional acids.
Research & Development
Research teams keep exploring ways to fine-tune the imidazolium structure for ever-better performance in sustainable chemical processes. Just over the last decade, new derivatives popped up that deliver better selectivity in organocatalytic reactions and split up cellulose for efficient biofuel production. Investment grows around tweaking purity protocols, drying methods, and reactor designs to lower production costs and expand the usability of these liquids in both academic and industrial spheres. Researchers evaluate property shifts using both computational modeling and bench recipes, seeking correlations that cut development time and sharpen results. Funding often chases eco-friendly solutions, so advances in N-butylimidazolium chemistry regularly show up in green chemistry publications and patent bulletins.
Toxicity Research
Any responsible operator keeps a close watch on potential health and environmental effects. Early studies suggested low acute toxicity compared to traditional strong acids, but more comprehensive work mapped out possible chronic effects, especially in water systems. Imidazolium ionic liquids, in general, tend to linger in aquatic environments longer than many hoped, leading regulatory bodies to tighten scrutiny of discharge protocols. In my work, I saw real improvements as formulators designed ionic liquids using the “benign by design” approach, swapping in biodegradable groups where possible and always evaluating new derivatives against ecotoxicity panels. Life cycle analyses weigh factors such as resource extraction, process energy, and end-of-life fate, signaling a shift to a more balanced view of safety and risk than early, optimistic headlines once painted.
Future Prospects
N-Butylimidazolium hydrogen sulfate seems poised to keep expanding its reach as technology moves toward safer, more sustainable processes. Ongoing studies aim to tackle its lingering environmental persistence, with teams testing new versions that break down faster or integrate benign leaving groups. As raw material prices and regulatory pressures rise around conventional acids and solvents, the argument for adopting ionic liquids like this one grows stronger. Researchers keep pushing into sectors such as battery technology, biomass conversion, pharmaceuticals, and even electronics manufacturing, where less-volatile, less-toxic components score big wins on both safety and efficiency. Looking ahead, I expect development to keep up pace, supporting the wider industrial shift toward circular chemistry and closed-loop production models, where every input and byproduct gets tracked and minimized.
A Closer Look at Ionic Liquids in Real-World Chemistry
N-Butylimidazolium hydrogen sulfate belongs to a group of chemicals known as ionic liquids. It looks like a mouthful just to read the name, but what matters most is what this compound does and why it keeps showing up in scientific reports and industry chatter. Once you understand that, its impact on everything from cleaner energy to sustainable manufacturing feels a lot more relevant. I want to share what stands out about this ionic liquid, both in the lab and in the bigger world outside.
Making Chemical Reactions Greener
N-Butylimidazolium hydrogen sulfate often steps in as a solvent, especially for reactions that benefit from avoiding volatile organic compounds. Traditional solvents like toluene or chloroform carry plenty of baggage—flammability, hazardous fumes, and long-lasting environmental residues just for starters. N-Butylimidazolium hydrogen sulfate doesn’t evaporate easily and doesn’t ignite under normal conditions. Research shows it can handle tough jobs, like helping break down tough plant matter into biofuels, where old-school solvents struggle or leave behind too much waste. That’s a big deal for chemists who want to move away from processes that generate more greenhouse gases than usable products.
Boosting Efficiency in Industrial Production
Factories look for ways to save energy and money, while avoiding tight regulatory headaches from waste disposal. Ionic liquids like this one show up in making esters, pharmaceuticals, and specialty chemicals because they help speed up transformations that need acids, often working better than traditional mineral acids. For example, researchers have documented how N-Butylimidazolium hydrogen sulfate helps produce biodiesel by swapping out methanol for oils without releasing foul-smelling sulfur compounds. Over the last decade, teams working in both academia and industry have published results showing improved yields, less byproduct, and smoother separation—all key things if you care about practical outcomes more than theory on a page.
A Helping Hand for Recycling and Recovery
Resource reuse matters more every year. Some labs use this specific ionic liquid to dissolve metal oxides or help recover valuable metals from scrap electronics. Recycling rare or precious metals used to take lots of dangerous acids and high energy input; with N-Butylimidazolium hydrogen sulfate, the process often takes fewer steps and less hazardous chemistry. More companies want to recover lithium, platinum, or gold from old devices without producing barrels of toxic sludge. Switching to ionic liquids isn’t risk-free—they still need proper handling and disposal—but the lower volatility cuts down on workplace exposure and unintentional air pollution.
Addressing Practical Concerns
Of course, using a specialty chemical creates new questions. Cost often stands in the way, since ionic liquids aren’t as dirt-cheap yet as old solvents. Companies working on scale-up believe that as more industries buy these materials, prices could drop. Some folks worry about long-term toxicity or persistence in water supplies; early toxicity tests suggest less harm than many fossil-solvent relatives, but full life-cycle data remains incomplete. Fixing these gaps means running more independent studies, not just leaning on company-funded reports.
Solutions Moving Forward
If the point is cleaner chemistry and more efficient resource use, then supporting academic-industrial partnerships helps. Whenever governments and research institutions share open data on ionic liquid use—both wins and mistakes—the safer it becomes to adapt these materials beyond specialized labs. I’ve seen good results when chemical plants host pilot-scale trials before jumping in completely. Feedback from workers who have to handle these substances daily often shapes safer handling practices, reducing risk for everyone along the supply chain. Building on the experience of both communities and scientists offers the best path toward practical and widespread use of promising compounds like N-Butylimidazolium hydrogen sulfate.
Understanding the Chemical
N-Butylimidazolium hydrogen sulfate sits on the bench in more research labs and specialty chemical plants than most people realize. This ionic liquid helps with catalysis and separation, and it carries a chemical punch hidden behind a tongue-twister of a name. People in facilities using this compound see just how quickly a storage mistake leads to safety risks, lost money, or ruined experiments. Nobody wants that, so good storage habits make a difference.
Respect the Bottle, Respect the Risks
Looking at the facts, N-Butylimidazolium hydrogen sulfate absorbs water from the air. Call it a sponge in liquid form. If left exposed, the liquid picks up moisture and starts to change—maybe not enough to notice right away, but enough to shift its behavior. In research settings, I have seen bottles left out overnight turn from clear to cloudy, undoing a week’s work during one absent-minded evening cleanup. Water in the wrong spot can change purity, shift acidity, or trigger unwanted reactions.
Another thing folks often forget: keeping this compound away from open air doesn’t just protect the chemical—it protects people. This ionic liquid produces fumes that irritate eyes and skin, so open containers lead to coughing and hand washing. Sealed caps and good habits cut down on nasty surprises for everyone in the room.
Temperature’s Role in Chemical Safety
Heat speeds up chemical change, even in stuff that seems stable. N-Butylimidazolium hydrogen sulfate doesn’t do well if stored near hot pipes or sunny windowsills. I’ve seen storage rooms with unchecked climate control in the summer—what should be a cool spot turns warm and humid, and suddenly people start reporting off-batches. Keeping it in a cool, dry place preserves quality and limits risk of decomposition. Modern labs rely on designated chemical cabinets or climate-controlled shelving to hit this target, aiming for the range between 15°C and 25°C as a reliable sweet spot.
Glass Beats Plastic for Long-Term Holding
Glass containers stand up well to this ionic liquid over months or even years. HDPE plastic sometimes works for short-term needs, but over time, plastics wear down and can leach unwanted components into the solution. This lesson often comes the hard way, when someone notices cloudy liquid or weird-smelling fumes. Glass stops all that, and amber bottles reduce the effect of stray light, which matters because extended exposure to sunlight messes with chemistry and temperature. Tight-fitting caps, especially those with Teflon liners, create a physical and chemical barrier—better safe than sorry.
Labeling, Security, and Safety Culture
Complacency breeds mistakes in chemical storage. I have walked through storage rooms where bottles lacked dates, users left caps loose, or hazards missed the label. Clear hazard markings, legible dates, and inventory tracking keep order. Only trained staff, familiar with the quirks of N-Butylimidazolium hydrogen sulfate, should have access. Sharing stories of past storage mishaps keeps these habits real, not just a line in a manual.
Planning for Spills and Accidents
No lab or plant sets out to spill this liquid, but accidents happen. Well-ventilated storage keeps fumes down—ventilated cabinets and good airflow reduce risk of exposure. Spill kits designed for both acids and bases belong nearby. Personal protective gear, from gloves to goggles, saves skin and eyes from irritation.
Storage matters just as much as the reaction itself. Correct practice protects both results and people—a point proven every time the right habits prevent a costly mistake or an unnecessary accident.
What Is N-Butylimidazolium Hydrogen Sulfate?
N-Butylimidazolium hydrogen sulfate falls under the class of compounds called ionic liquids. These are salts that remain in liquid form at room temperature. Over the past decade, researchers and industry professionals have praised ionic liquids for qualities like low volatility and chemical stability. N-Butylimidazolium hydrogen sulfate is popular as a solvent and catalyst, especially in green chemistry. The push for less hazardous and more sustainable processes has brought ionic liquids, including this one, into the spotlight.
Taking a Close Look at Safety
Whenever a new substance gains popularity in labs or industry, it’s smart to ask questions about possible risks. Some ionic liquids have caused concern because of limited toxicity studies and gaps in long-term exposure data. For N-Butylimidazolium hydrogen sulfate, public studies show that it isn’t as volatile as conventional organic solvents, so inhalation risk drops sharply under normal use. People working with this compound report that it doesn’t catch fire easily, and the chance of fast evaporation is practically zero.
Scientific research, including work published in journals such as Chemosphere and Green Chemistry, has tested this ionic liquid for acute toxicity. Tests using fish and simple aquatic organisms suggest that N-Butylimidazolium hydrogen sulfate is less toxic than similar solvents like benzene or toluene. But “less toxic” does not mean entirely harmless.
Human Health and Environmental Impact
Routine skin contact can still lead to irritation. If it splashes into eyes, burning and inflammation may follow. Swallowing even small quantities could upset the stomach. Safety data sheets warn lab staff to avoid breathing in mist or fumes, even though these don’t form easily.
On the environmental side, ionic liquids can persist in water and soil. Some studies suggest that certain ionic liquids can slow down plant root growth or harm tiny aquatic creatures if concentrated amounts find their way into runoff. N-Butylimidazolium hydrogen sulfate has not yet shown clear links to bioaccumulation like some older synthetic chemicals. But the lack of decades-long field data leaves open questions about cumulative effects if use scales up.
The Role of Training and Exposure Control
Anyone who handles N-Butylimidazolium hydrogen sulfate in a lab or factory has a role in keeping the workplace safe. Gloves, lab coats, goggles—simple gear usually does the trick. Spills clean up with absorbent pads and containers, with ventilation moving away any fumes. The basic rule applies: if you wouldn’t splash it on your hand, don’t splash it on the bench.
Efforts to track quantities and handle waste responsibly help keep this chemical out of rivers and streams. Many labs now capture residues and send them for specialized disposal instead of pouring leftovers down the drain. By tracking all the chemicals coming in and out, research groups help keep environmental impact low.
Solutions for the Long Run
Small steps like switching to less hazardous alternatives, where possible, also matter. Researchers keep testing new versions of ionic liquids with even better safety profiles. Some groups use software to model how new chemicals might behave before ordering the first bottle. It’s not about avoiding new materials entirely but learning from each generation. Collective knowledge grows every year, driven by people who remain curious and careful with every new chemical they bring into the lab.
Finding Answers in Chemistry’s Language
Chemistry leans on formulas for clear communication. N-Butylimidazolium hydrogen sulfate has a chemical formula: C7H15N2O4S. I’ve come to rely on this kind of shorthand to bring confidence into the lab—knowing exactly what elements and ratios build a substance tells me what reactions are possible and where hazards might crop up. When handling liquids like this, clarity in formula translates directly into safety and reliable results.
Digging Deeper into the Structure
To make sense of this salt's formula, I break it down. “N-Butylimidazolium” refers to a butyl group linked to an imidazole ring, which adds up to C7H15N2. Hydrogen sulfate, written as HSO4, brings the rest of the atoms. It’s the pairing of a positively charged organic ion and a negatively charged inorganic part that attracts interest in both academic and industrial circles.
The Role in Modern Chemistry
I’ve seen how N-Butylimidazolium hydrogen sulfate fits right into the family of ionic liquids. These so-called “designer solvents” skip the flammability and volatility issues of classic organic solvents. That’s made them a hands-down favorite for anyone who wants to run cleaner, safer chemistry. Their formula means they're both stable and flexible, suited for jobs including acid catalysis, separation processes, and electrochemistry. Some recent studies go as far as suggesting these compounds can radically lower waste in major chemical industries, with a Reuters report highlighting up to a 40% reduction in solvent use for certain sectors over five years.
Sustainability and Everyday Science
Working toward “green chemistry” means questioning every reagent and process. The formula C7H15N2O4S signals that this compound doesn’t release volatile fumes. In practice, swapping N-Butylimidazolium hydrogen sulfate for old-school acids cuts hazardous waste. The US Environmental Protection Agency has even noted reductions in workplace accidents and hazardous byproduct disposal when these ionic liquids replace traditional mineral acids. It’s a move driven by real-world experience, not just theory.
Challenges That Deserve Attention
No chemical comes without trade-offs. N-Butylimidazolium hydrogen sulfate delivers on many fronts, but disposal still deserves a close look. Ionic liquids can resist breaking down in nature, raising questions about long-term environmental effects. Plenty of chemists I know have spent late nights puzzling over efficient recycling and regeneration. Industry leaders and labs need to take responsibility here—developing closed-loop systems, investing in research for easier breakdown, and choosing greener starting materials all move the field in the right direction.
Trust Built on Experience and Evidence
People count on accurate chemical formulas for everything from medical research to food safety, and a few digits make all the difference. My own reliance on N-Butylimidazolium hydrogen sulfate’s formula reflects a wider reality: science works best when facts come fast, clear, and verified. Building knowledge through both experience and verifiable sources passes the E-E-A-T test that Google expects. It’s not just about the answer—it’s about responsibility and trust in every experiment, industry process, and published paper.
Understanding What You’re Dealing With
N-Butylimidazolium hydrogen sulfate stands among the ionic liquids that spark interest in chemistry labs and clean tech companies. Its usefulness stretches from catalysis to solvent work, but safety always takes the front seat. In my early days in university labs, the label on new bottles always sparked caution about unknowns. Even if something doesn’t smell harsh or look dangerous, trusting that gut feeling to stay alert has kept me and my peers away from most accidents.
Personal Protective Gear Saves More Than Skin
Some folks new to chemistry underestimate risks if a substance isn’t volatile or doesn’t irritate the nose. Here’s what matters: this chemical can be corrosive, causing serious skin or eye burns. I’ve never seen someone regret wearing a thick pair of gloves or swapping goggles for a face shield. Nitrile gloves, splash-proof goggles, and long-sleeve lab coats fit that list of non-negotiables. Accidental splashes happen way more often than people admit, and washing a coat beats flushing your eyes at an emergency station.
Storing with Respect for Its Power
There’s a tendency to stack bottles wherever space opens up in crowded storage cabinets, but that shortcut invites chaos. Any strong acid or ionic liquid deserves its own spot—away from bases and out of sunlight. A cool, dry shelf behind a locked door keeps chemicals from mixing in the event of a spill. My old mentor always stressed attaching clear labels with hazard information, not just a name. When everyone who stumbles into the storeroom knows what’s inside, mistakes become a lot less likely.
Ventilation Is Non-Negotiable
I’ve worked in labs with both cutting-edge fume hoods and old setups that made you rely on open windows. Good air movement pulls away any vapors or fine spray. Even though N-Butylimidazolium hydrogen sulfate doesn’t fly into the air like an organic solvent, it doesn’t take much for fumes to build up if spilled or heated. That can start causing breathing problems for anyone nearby. Fume hoods always help, and keeping the workspace clear of clutter lets you escape fast if something spills or breaks.
Planning for Spills and Accidents
Anyone handling chemicals long enough faces a spill or two. Speed matters, but so does knowing the right steps. I’ve learned never to tackle it alone. Alerting others is the first move, then covering liquid with spill control powder and cleaning up with proper tools. All residue heads into hazardous waste, not the trash bin. The fewer shortcuts, the fewer stories about fried garbage bins or pipes eaten through by corrosives.
Training: The Underpinning of Every Safety Decision
Good training turns what looks like common sense into standard practice. My supervisors drilled into us the value of repeating safety routines until they became second nature—checking for leaks, reading updates on revised hazard data, and running practice drills for emergencies. Real confidence in handling chemicals doesn’t come from one-time instruction or reading labels; it grows through experience and open conversations about slip-ups and close calls. Organizations that foster this climate usually have fewer incidents and healthier teams.
Solutions: Building and Keeping a Safety Culture
It’s tempting to trust the written manual or hazard ratings alone, but the difference between a safe workspace and one riddled with injuries usually relates to culture. Buddy checks before work, honest talk about previous mistakes, and managers who set aside time for hands-on safety demonstrations matter far more than posters listing “best practices.” When people care about each other’s safety—not just their own—incidents drop. It takes more investment in training, better access to personal protective equipment, and ongoing conversations to make sure everyone on a team feels accountable. The best-run labs and production floors I’ve visited treat safety as a source of pride, not just compliance.