N-Butylimidazolium Tosylate: A Down-to-Earth Look at a Modern Ionic Liquid
Tracing the Story of N-Butylimidazolium Tosylate
Digging into the history of N-butylimidazolium tosylate gives us perspective on how chemistry has changed. This compound’s roots run close to the rise of ionic liquids, which chemists first viewed as exotic substances with unusual powers. Over the past few decades, the serious push for cleaner and more efficient chemical processes gave ionic liquids real momentum. Chemists started tinkering with imidazolium cores around the 1970s, but it wasn’t until the late 1990s that practical versions like N-butylimidazolium tosylate began to show up in labs and industries. As the urgency for "green chemistry" keeps climbing, people keep turning to molecules like this for answers. Watching big chemical manufacturers invest in ionic liquids, and seeing patent applications involving these structures expand every year, shows there’s now some staying power behind earlier hype.
Breaking Down What N-Butylimidazolium Tosylate Is
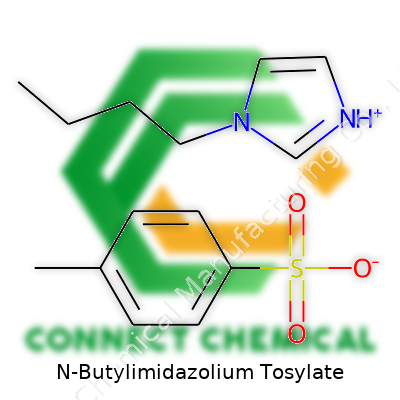
At its core, N-butylimidazolium tosylate has an imidazolium ring stuck to a butyl group on one side, paired up with a p-toluenesulfonate anion. To anyone who’s spent time in the lab, this structure feels both familiar and fresh. You get the thermal stability of imidazolium, the tunability of alkyl groups, and a well-behaved sulfonate. Its solid or viscous nature depends on subtle things like temperature or impurities from preparation. In practical experience, you pour it or scoop it, sometimes sticky—always with a mild, not-unpleasant organic scent.
Physical & Chemical Properties
Long hours in the lab teach you to look for what matters: how a chemical moves, behaves, and smells. N-butylimidazolium tosylate, with a melting point hovering anywhere from low 60s to the mid 70s Celsius, sits just between a true solid and something soft enough to stir. It loves water but isn’t greedy. This ionic liquid handles air decently; you won’t lose it to evaporation unless conditions get wild. Viscosity runs high, especially compared to common solvents like acetone or ethanol. The compound’s density usually feels heavier in a vial—evidence of ionic particles packed close. Thermal stability stands out; you can push it over 200°C before you worry about real decomposition. As for solubility: It breaks rank with most imidazolium salts by cooperating well with both polar organic solvents and, to a lesser extent, water.
Technical Specifications and Labeling
Anyone working with chemicals knows the value of clear information. Vendors sell N-butylimidazolium tosylate in various purity grades, with most research-grade materials boasting >98% assay, low moisture, and strictly defined color (often faint yellow to colorless). Bottle labels show the chemical’s full systematic name, CAS number, batch code, and shelf life—all necessities for anyone tracking standards or regulatory requirements. Major suppliers often attach technical data sheets with in-depth reports: melting point ranges, elemental analysis, NMR spectra, and so on. For all that, buyers often double-check important things like halide content, residual solvent levels, and decomposition temperature, especially if the compound heads into sensitive synthetic or catalytic applications.
Making N-Butylimidazolium Tosylate: The Preparation Routine
Preparation stands as both art and science. The standard route involves alkylation of imidazole with butyl halide, using a base, often at mild temperature, then exchanging the halide for tosylate by reaction with sodium or potassium p-toluenesulfonate. Some old-timers swear by meticulous purification at each step: careful washing, filtration, and sometimes repeated recrystallization. Skipping corners often leads to ugly results—impure products cause unreliable lab data and, worse, failed industrial runs. In my experience, you can predict a batch’s behavior almost entirely by rigor in preparatory steps. For larger operations, continuous-flow setups streamline salt metathesis, minimize solvent waste, and shave off labor costs, showing how process chemistry can bridge the gap between bench and plant floor.
Chemical Playgrounds: Reactivity and Modification
N-butylimidazolium tosylate isn’t a one-trick pony. The cation can shuffle electrons across its ring system, making this salt useful in phase-transfer catalysis, as a solvent for metal complexes, and even a supporting electrolyte in specialized electrochemistry. The butyl side-chain opens doors for further chemical grafting—swapping other alkyl chains, adding branches, or even sticking in polar groups for targeted performance. The sulfonate anion resists oxidation, standing up to some harsh reaction conditions. Experimental chemists often remix the cation or swap out the anion entirely, chasing efficiency in separations or catalysis. In the field, this leads to modified salts designed for task-specific uses: from drug delivery systems to stubborn polymerizations.
Synonyms and Product Names
Shopping for this compound at chemical supply houses brings home just how many alternate names float around. You’ll see labels like 1-butyl-3-methylimidazolium tosylate, BMIM-TsO, or just [BMIM][TsO]. I’ve also gotten bottles labeled with the full IUPAC spelling: 1-butyl-3-methyl-1H-imidazol-3-ium 4-methylbenzenesulfonate. All these names point to the same molecule, but confusion isn’t rare. People often identify the product by its ionic pair or shorthand, especially in technical meetings or procurement departments.
Safety & Operational Standards
Folks sometimes forget that ionic liquids, despite their "green" image, deserve respect in the lab. N-butylimidazolium tosylate doesn’t explode or burn easily, but skin contact can bring mild irritation for sensitive users. Lab SOPs call for gloves, goggles, and splash protection; accidental eye exposure might sting but doesn’t cause lasting damage most of the time. Handling this chemical on production scale involves local exhaust, careful storage, and spill containment. Disposal flows into the general rules for organics, avoiding drain dumping, favoring incineration, and adhering to local environmental laws. Some suppliers started offering alternatives in smaller, more user-friendly packaging to cut the chance of workplace mishaps.
Use Cases in Real Work
Across research and industry, N-butylimidazolium tosylate fills a growing list of roles. I’ve seen it act as a solvent in Suzuki and Heck coupling reactions, and it cuts down on toxic by-products while boosting selectivity. Labs use this ionic liquid for separation of lignocellulosic biomass, extraction of rare metals, and protein crystallization. Battery developers and electrochemists run tests on its conductivity, hoping for breakthroughs in energy storage. Drug scientists like this compound for its ability to dissolve both hydrophilic and hydrophobic substances, opening fresh possibilities for pharmaceutical formulations. Pilot plants value its low vapor pressure—almost no loss to air, translating to safer processing and lower emissions.
Research & Development Momentum
A quick sweep through recent journals and patent records shows real momentum driving innovation with this salt. Materials scientists are embedding N-butylimidazolium tosylate into polymer electrolytes, searching for robust, flexible membranes. Green chemistry teams engineer catalytic cycles that leverage its polarity and stability, trying to clean up traditional reactions that stink of heavy metals and leftover solvent. Bioseparation specialists exploit its customizability in extracting delicate biomolecules from tough mixtures. The compound’s versatility keeps research streams active: new salt combinations, custom viscosity control, and hybrid ionic liquid systems put this chemical at the front of several "next generation" initiatives.
Toxicity Research: Keeping Promises in Check
The “environment-friendly” tag on ionic liquids sometimes crumbles under scrutiny. Labs have tested N-butylimidazolium tosylate for acute toxicity in aquatic creatures, and while it doesn’t behave like conventional industrial pollutants, some studies show it slows growth or hinders reproduction in sensitive species. Its breakdown in nature creeps along at a slow rate, especially in soils, so its buildup in water-rich environments draws some worry. Regulatory authorities demand detailed material safety data and, recently, insist on new eco-toxicological screens with each new batch. Developers pursuing scale-up for pharma or agrochemicals often build in multiple separation or degradation steps to trap residues before final disposal. It’s clear this chemical isn’t a panacea—inspection and thoughtful management set a real example for sustainable industrial chemistry.
Possible Directions for the Future
N-butylimidazolium tosylate, after standing the test of early hype, now faces real evaluation in industry. Performance claims meet hard financial and safety tests as more companies trial ionic liquids against incumbents like NMP or DMSO. Next-gen batteries need electrolytes that balance power, stability, and cost; those trade-offs play out in ongoing studies. Environmental pressure grows as green chemistry shifts away from mere “less hazardous” to full lifecycle scrutiny, pushing manufacturers and researchers to prove this salt’s downstream fate. Whether as a laboratory workhorse or a catalyst in greener factories, N-butylimidazolium tosylate’s future will depend on honest handling of its risks, ongoing process innovation, and continued open research. For now, its adaptability, proven performance, and ease of modification make it a fixture in labs and pilot plants, but only clear-eyed stewardship keeps it that way.
A Peek Inside the Lab: Real Uses for N-Butylimidazolium Tosylate
Walk into any modern chemistry lab and you’re likely to spot ionic liquids lined up like bottles on a bar cart. N-Butylimidazolium Tosylate pops up more often than you’d think. It’s got a funny-sounding name, but chemists turn to it for a handful of good reasons. People talk about ionic liquids as if they reinvented the wheel, but let’s keep it real—most folks running experiments are on the hunt for safer, cleaner, more efficient tools. That’s exactly where this compound shines.
Solvent That Stands Out
N-Butylimidazolium Tosylate pulls its weight as a solvent. Traditional solvents like toluene or acetone evaporate quickly and bring their own baggage—flammability, health risks, and a mess of regulations. This ionic liquid throws in a higher boiling point and low vapor pressure. In plain English: it’s less likely to catch fire, and you won’t see clouds of vapor escaping into the room. Less stink, less stress. Anyone working through a hot summer week stuck indoors can appreciate that difference.
Green Chemistry Is Not Just a Buzzword
Green chemistry tries to fix some of the ugly problems regular labs cause. I’ve watched enough beakers get poured down a drain to know sustainability isn’t just about lab coats making statements for a grant. N-Butylimidazolium Tosylate helps reactions run at lower temperatures. Less energy spent on heating means a smaller electric bill. The cation and anion make it more stable, so it doesn’t break down and clog up your product with side junk. This saves time during clean-up and actually helps companies cut waste disposal costs. Better for the planet and better for the bank account.
Performance in Catalysis
Ionic liquids step up enzyme reactions, and this one is no exception. Enzymatic processes get lazy in water or suffer in alcohols. N-Butylimidazolium Tosylate gives enzymes a bath where they stretch out and work harder—better yield, cleaner end result. When I ran biocatalysis tests with and without this stuff, side-products dropped, and my supervisor stopped giving me the side-eye over inconsistent data. Published studies have pointed out that it can also anchor palladium and other metal catalysts, keeping them in place and recycling them afterward. Fewer metals tossed, lower costs, and quicker lab turnarounds. These improvements get noticed in pharmaceutical plants, not only university benches.
Pushing into New Technologies
There’s a race to make batteries worth their weight. Battery developers reach for safer electrolytes and ionic liquids with high thermal stability. N-Butylimidazolium Tosylate fits that mold. Used in prototype lithium-ion and dye-sensitized solar cells, it boosts conductivity without risking battery fires like volatile liquids do. Energy innovation needs building blocks that don’t blow up under pressure, and every bit of safety helps scale up projects beyond the prototype stage.
How to Handle the Hurdles
Chemists need to watch out for purity. Any leftover starting material or water can drag down results. Labs monitor cost, too—while ionic liquids look pricey up front, their longer lifespans and reusability often pay back the investment. While nobody claims it’s the final answer for every reaction or battery breakthrough, real-world experience shows it does far more than just sit in a reagent drawer. N-Butylimidazolium Tosylate lets researchers breathe easier—literally and professionally—while nudging science toward cleaner, safer futures.
Getting Familiar With a Smart Solvent
N-Butylimidazolium Tosylate doesn’t usually make the front page, but this ionic liquid shows up everywhere in the world of green chemistry and industrial research. On the lab bench, the stuff catches the eye because it pours slowly, almost syrup-like, with a clear to pale yellow look. You often find it at room temperature as a viscous liquid. If you heat it, it handles high temperatures without breaking a sweat, staying thermally stable up around 200°C or more. This means it keeps its cool through processes that would knock traditional solvents out of the running.
Breaking Down Its Chemical Attitude
At the molecular level, the butyl group hanging off the imidazolium ring gives it real muscle for dissolving a range of compounds, both polar and non-polar. I’ve watched it take up metal salts, cellulose, and even dyes that barely mix in water or standard organics. The tosylate part, coming from toluenesulfonic acid, adds a touch of bulk and makes the whole compound friendly toward organic transformations. This specific pairing of cation and anion means you dodge volatility—no noticeable smell and practically zero vapor pressure, which as a chemist, lets you breathe easier and avoid explosions or fire.
Handling and Real-World Experience
Lifting a bottle of N-Butylimidazolium Tosylate for the first time, you’d notice its weight compared to water—the density actually sits a bit higher, around 1.2 g/mL. Accidental spills show that it clings and leaves a slippery patch, so you want gloves and immediate cleanup. Despite this, it’s relatively straightforward to store, avoiding the ether-like headaches most solvents deliver.
Why Chemists Value It
In the lab, people lean on this ionic liquid for its extreme stability and tailor-fitted solubility. Its ionic nature means solutions conduct electricity—useful for certain electrochemical setups or battery research. The high boiling point helps in extractions that run at higher temps, or for trying to pry valuable compounds out of tough materials, like biomass. Its tolerance for moisture lets you skip some tedious drying steps that slow traditional syntheses. Research published by groups studying green synthesis highlights less hazardous waste, giving N-Butylimidazolium Tosylate some environmental street cred.
Hurdles to Wide Adoption
No chemical escapes drawbacks. Disposing of ionic liquids creates a waste challenge, since they won’t evaporate off a benchtop or break down quite like alcohols or acetone. Price tags hit harder than on everyday solvents. Purity can swing between suppliers, so analytical labs sometimes hit problems running repeat experiments. Regulatory questions still float over broader use, especially for pharmaceutical applications where toxicology data needs more filling out.
Looking Toward Better Solutions
Earning a spot in everyday chemistry won’t happen until the industry solves waste treatment and resource recycling. More companies are tackling recovery systems using filtration, distillation, or even supercritical CO2 to reclaim and reuse these ionic liquids. I’ve seen groups experimenting with biodegradable variations, swapping pieces of the molecular structure for greener parts. Sharing rigorous purity data helps researchers and producers keep everyone’s results a little less unpredictable, which matters when a single contaminant derails a day’s worth of work.
The Impact of Knowledge and Use
For those mixing up new reactions or scaling sustainable methods, N-Butylimidazolium Tosylate gives fresh options. Advanced properties open doors for less wasteful, safer, and sometimes more efficient chemical processes if labs and manufacturers can keep up with ongoing research. Keeping an eye on both the opportunities and hurdles lets anyone making, using, or regulating this substance drive progress for the entire field.
The Nature of N-Butylimidazolium Tosylate
N-Butylimidazolium Tosylate serves as a key ionic liquid in many labs. I remember my first time working with it: instructions from the lab manager sounded simple, but in practice, careful steps made all the difference. Above all, safety measures protect the people closest to these chemicals. N-Butylimidazolium Tosylate can irritate the skin and eyes, and inhaling dust poses risks too. Neglecting basic hygiene leads to problems quickly, so a culture of care belongs in every setting where chemicals appear.
Solid Storage Habits
Every bottle in the cabinet says something about the habits of a lab. This one stays best in a tightly sealed container, away from sunlight and heat. I always check the temperature in the storage room—it ought to stay cool and dry, out of reach of humidity. Water finds its way into the tiniest cracks, and letting in any moisture changes the compound or produces clumps that become hard to measure. Choosing glass or high-quality plastic bottles with tight-fitting lids stops accidental spills and drips. Colored bottles help keep the light away, which keeps the compound from degrading.
Storing it on lower shelves, not above eye level, can prevent nasty accidents. Accidental drops lead to chemical exposure and broken glass, making a mess that no one wants to clean up. A clear label in bold print helps everyone avoid confusion with other, similar-looking chemicals. In my lab, a printed chart sits at eye level on the door listing hazardous substances and first aid reminders. Little steps like this add layers of safety.
Handling Precautions Save Skin and Eyes
I have seen what happens when people skip gloves because they’re in a hurry. Proper gloves, goggles, and lab coats won’t slow anyone down. Touching bare skin with ionic liquids leads to irritation or more severe reactions, depending on the person. For most tasks, nitrile gloves provide solid protection. Eye shields, not just prescription glasses, block splashes that can burn or damage vision for life. Wearing a lab coat and working in a ventilated fume hood also reduces risk.
If a spill happens, paper towels won’t cut it. Having special absorbent materials ready matters. It’s worth placing a chemical spill kit nearby, topped with a set of step-by-step instructions. If N-Butylimidazolium Tosylate touches skin, flushing with flowing water for several minutes buys valuable time. Eyes demand urgent rinsing at an eyewash station—no exceptions. Every year, practice drills remind us where the closest shower and eyewash units stand. Quick action can change the outcome.
Waste Handling and Clean-Up: A Team Effort
No one likes dealing with chemical waste, but everyone must do it right. Used N-Butylimidazolium Tosylate shouldn’t go into the sink or the regular trash. My routine involves sealing waste in a labeled, leakproof container and logging it for scheduled hazardous waste pickup. Uncapped bottles fill the air with fumes and invite accidental spills. Any contaminated rags or gloves go into marked chemical waste bins. Clean work surfaces and frequent handwashing help everyone stay safe, whether working alone or as a team. Sharp focus on these small routines keeps the harsh surprises away.
What N-Butylimidazolium Tosylate Does in the Lab
N-Butylimidazolium Tosylate is part of a group called ionic liquids. Thanks to its special chemical structure, scientists lean on it a lot to dissolve tough stuff or speed up reactions in the lab. People working in pharmaceuticals, chemical research, and green chemistry find it pretty handy because it makes work faster and a little less wasteful compared to old-school solvents. That popularity brings up a real question: can using it harm our health or surroundings?
Weighing the Risks: What Science Says About Hazards
Toxicology research on N-Butylimidazolium Tosylate is pretty new. Still, what has come out so far rings a few bells. Some ionic liquids can mess with living cells, either slowing cell growth or causing outright damage, especially if they're handled carelessly or dumped. Scientists from research centers in Europe flagged cell toxicity in different animal studies. The detail that makes this more pressing comes from the way ionic liquids can travel in water, potentially reaching soils and streams if not treated right. Animals and aquatic plants show higher sensitivity to these chemicals, with some fish and algae reacting at surprisingly low concentrations.
Not all ionic liquids act exactly the same way, and N-Butylimidazolium Tosylate doesn’t stand alone here. Its butyl chain and tosylate anion give it properties that let it behave differently under heat or pressure. These traits also affect how it interacts with living tissue and the environment. So, the whole picture depends on how it's used, stored, and disposed of in practice.
Handling and Workplace Safety
Lab workers using N-Butylimidazolium Tosylate need strong habits to protect themselves. Gloves, eye protection, lab coats, and fume hoods stop accidents before they happen. Inhaling or spilling this liquid on your skin can irritate and possibly lead to longer-term health problems. Because full safety data is still growing, companies and research groups take the cautious route, treating this compound with the same care they'd use for more obviously toxic substances.
There’s a lot of experience from decades spent with ionic liquids. Most labs train up young chemists to respect unknowns, not just what the labels say, since new chemicals keep showing up. My own time in university research taught me to follow strict safety routines, always log chemical waste, and wash hands after even minor handling. Even with gloves, I learned to stay aware of accidental contact—those few times someone ignored a splash led others to tighten up safety rules quickly.
Is It Safer Than Older Solvents?
Some people call N-Butylimidazolium Tosylate a “green solvent.” This idea grabs headlines. In reality, “green” just means it doesn’t evaporate into the air as fast or pollute ground water as much as traditional solvents like benzene or toluene. That’s a step forward. On the other hand, ionic liquids break down pretty slowly. Once spilled, they can linger in the environment for a long time, making proper waste management a must.
Protecting People and the Environment
Regulation lags a bit behind the science with compounds like this. Until clear official guidelines appear, the safest route means careful labeling, good chemical hygiene, and full disposal records. Researchers also push for better testing and more open reporting on side-effects. This helps regulators and companies build smarter policies and even tailor better solvents down the road.
N-Butylimidazolium Tosylate pulls its weight in science, but it deserves watchful use. Experience, up-to-date research, and smart policy build the foundation for safe work, cleaner industries, and a healthier environment.
Why N-Butylimidazolium Tosylate Matters in the Lab
Ask a chemist for a handy, versatile ionic liquid, and many will rattle off N-Butylimidazolium Tosylate among their favorites. Labs use it because it handles heat well and resists volatility, making it great for tricky reactions that struggle with harsher solvents. Synthetic chemists lean on it when working on green chemistry, aiming to reduce the environmental punch that comes with most organic solvents.
One standout feature is its knack for dissolving a wide mix of chemicals, especially those that refuse to budge in water or regular solvents. On my bench, I’ve seen teams turn to N-Butylimidazolium Tosylate in Suzuki coupling, Heck reactions, and other metal-catalyzed processes. Its role? Acting as both solvent and stabilizer, it helps metals stay available for longer, which often means bigger yields and easier post-reaction clean-up. The result isn’t just lab convenience – it’s a pretty strong argument when you need to convince the purchasing department that green chemistry can also save money.
Real Uses on the Factory Floor
N-Butylimidazolium Tosylate doesn’t stop at small-scale science. In industry settings, folks running catalytic processes appreciate its heat and chemical stability. It gives cleaner splits between product and waste, so you spend less time and energy on separation, then score purer output at the end.
The pharmaceutical industry is a good example. Drug manufacturers put a premium on reactions that can go smoothly and cut down on contamination. Using this ionic liquid helps keep catalysts in play for longer, slashes metal contamination in the final product, and makes purification less of an ordeal. In the dye and pigment field, companies tap this liquid for more even reactions and better control over color quality.
Cleaner, Simpler Extraction and Processing
Extraction specialists talk a lot about getting more without using harsher chemicals or raising costs. N-Butylimidazolium Tosylate often comes up in extraction of bioactive compounds, especially in food and plant-based research. Its ability to dissolve both water-loving and oil-loving molecules means researchers can extract flavors, fragrances, or nutrients from plants without dragging along unwanted byproducts.
In my own experience, I’ve tried using it to pull antibacterial compounds from herbs, with better results than standard alcohol or oil extractions. The benefit? Less reliance on petroleum-based solvents, a safer process for lab workers, and fewer headaches in waste disposal.
Tackling the Criticisms
No tool is perfect, and some critics worry about recycling or disposing of ionic liquids. The cost of N-Butylimidazolium Tosylate runs higher than bulk solvents like ethanol, so companies seek ways to recover and reuse it on site. In my group, we’ve had success with simple distillation or membrane separation, getting 80% or more back in usable form.
For those concerned about toxicity, it helps to remember ionic liquids have widely differing safety profiles. N-Butylimidazolium Tosylate tends to be less persistent and more biodegradable than older alternatives, but researchers and safety committees still keep its handling under review.
What Can Improve Moving Forward?
Wider adoption hinges on a few things: better recycling systems, lower prices through streamlined production, and greater education around safe use. The trend of switching to greener solvents is building momentum. If producers and users invest in best practices for recovery, N-Butylimidazolium Tosylate could see even broader use in both research and industry. To get there, clear communication between lab scientists and plant managers will make sure the full benefits kick in, with less worry over waste or cost.