N-Butylimidazolium Trifluoromethanesulfonate: A Deep Dive into Modern Chemistry
Historical Development
Research into ionic liquids began in earnest during the late 20th century, mostly driven by a need for stable, non-volatile solvents that could operate under demanding conditions. Chemists hunting for safer options than classic organic solvents stumbled upon imidazolium-based salts. The introduction of N-Butylimidazolium Trifluoromethanesulfonate marked a breakthrough, offering a significant step away from environmental hazards posed by volatile organic compounds. Laboratories in Europe and Asia contributed much to the early work, aiming for compounds that could handle heat, resist chemical wear, and speed up reactions in ways that old-school solvents rarely managed.
Product Overview
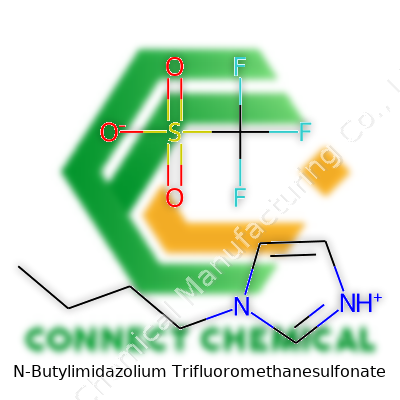
N-Butylimidazolium Trifluoromethanesulfonate stands out as an ionic liquid, part of a family celebrated for non-flammability and low vapor pressure. The butyl side chain gives it unique flow properties, making this chemical valuable for applications ranging from electrochemistry to catalysis. Labs gravitate toward its reliable consistency and performance when dealing with high temperatures or seeking greener alternatives to traditional solvents.
Physical & Chemical Properties
This material typically appears as a colorless to pale yellow viscous liquid at room temperature. Density reaches about 1.3 g/cm³, while melting points hover well below typical lab temperatures, keeping it fluid through a wide range. The combination of the imidazolium cation and the trifluoromethanesulfonate anion gives the compound remarkable thermal and chemical stability, which enables it to outperform-molecular solvents in endurance tests. It dissolves salts and polar compounds with surprising ease, driving large scale interest in process optimization and green chemistry projects.
Technical Specifications & Labeling
Chemical suppliers usually mark up N-Butylimidazolium Trifluoromethanesulfonate with its CAS registry number and product codes for tracking and quality assurance. Purity hits 98% or greater in most commercial samples. Residual water content tends to stay below 0.2%, which matters for electrochemical uses where extra water can interfere with reactions. Labels feature detailed storage info, including recommendations to keep containers tightly sealed and out of bright light, given its mild sensitivity to atmospheric moisture and certain environmental factors.
Preparation Method
Chemists synthesize N-Butylimidazolium Trifluoromethanesulfonate through an alkylation route that joins N-methylimidazole with butyl halide, followed by anion exchange using trifluoromethanesulfonic acid. Reaction setups call for attentive temperature control and inert atmospheres to avoid side reactions. After filtration and washing, purification through vacuum drying brings the ionic liquid up to specifications. Each step demands solid process experience, as impurities can hamper final properties and downstream performance.
Chemical Reactions & Modifications
Experiments show this ionic liquid holds up in the presence of many reactive metals and acids, rarely breaking down even after repeated thermal cycles. Researchers often use it as a solvent for organic transformations, where it doesn’t strip functional groups or leave tricky residues. On occasion, modifications to the side chain or swaps of the sulfonate for another stable anion let chemists tailor properties for very specific jobs, like separating challenging mixtures or enabling greener battery architectures.
Synonyms & Product Names
On the shelf or in literature, this compound sometimes turns up as 1-Butyl-3-methylimidazolium trifluoromethanesulfonate or BMIM triflate. Synonyms help users spot equivalent products distributed under brand or regionally specific catalog codes.
Safety & Operational Standards
Strict protocols cover everything from handling to waste disposal. Although N-Butylimidazolium Trifluoromethanesulfonate avoids the volatility of classic solvents, it can irritate skin or eyes upon direct contact, and accidental inhalation should be avoided due to possible respiratory irritation. Personal protective gear—including gloves and goggles—remains a fixture in labs using this liquid. Hazard communication and spill response plans mean risks drop to manageable levels; safe operation relies on experienced users treating each step as a chance to avoid mishaps.
Application Area
Electrochemical studies benefit from this liquid’s high conductivity and stability, with performance boosting redox reactions and battery prototypes. In organic synthesis, it gives chemists a greener, reusable platform—unlike single-use halogenated solvents, this one can often undergo recovery and reuse cycles. Material scientists pursue it for dissolving polymers, or as a medium for producing nanoparticles with even size distribution. Some startups even investigate its use in gas separations and carbon capture, banking on its ability to hold up where traditional compounds fall apart.
Research & Development
Innovation grows steadily around N-Butylimidazolium Trifluoromethanesulfonate. The number of publications continues rising as scientists test out both old and emerging reactions in its medium. Cross-disciplinary teams share stories of projects that cut waste output and reduce hazardous by-products by 80% or more, especially in academic settings where proving environmental safety ranks high. Partnerships between manufacturers and academics foster iterative improvement, moving beyond simply selling the material to actively tweaking formulas for best performance.
Toxicity Research
Studies so far point out low acute toxicity for this ionic liquid, with chronic effects just starting to get close scrutiny. Environmental fate draws real concern; while low volatility lessens air emissions, degradation in soil or water can be slow. Experiments for aquatic toxicity reveal moderate impacts on invertebrates, pushing regulatory agencies to watch discharge and waste treatment more closely. Chemists believe that with further modification, eco-friendliness stands a fair chance of improvement, but users see value in limiting unnecessary exposure and keeping up to date on regulatory changes.
Future Prospects
As the world looks for solvents that meet both performance and environmental targets, interest in N-Butylimidazolium Trifluoromethanesulfonate grows. Emerging battery research and chemical engineering both call for precisely these properties. Broader adoption hangs on price drops and innovations in production scalability. Young researchers see untapped potential in areas like biocatalysis, corrosion protection, or smart materials, and investment in pilot programs brings new solutions into focus. There’s a mood of optimism among those who see the shift toward greener chemistry as vital, and this compound stands up as one valuable tool on that path.
What N-Butylimidazolium Trifluoromethanesulfonate Does
N-Butylimidazolium trifluoromethanesulfonate lands on lab benches as a room-temperature ionic liquid. Its unique mix of organic and inorganic features draws attention in chemistry circles, especially among those searching for efficient and safer alternatives to harsh industrial chemicals. Scientists and engineers see potential here beyond just one field.
Green Chemistry and Better Solvents
Traditional solvents come with real downsides—think fire risks and toxic fumes. This ionic liquid skips those headaches. Its stability matters. In organic synthesis, switching to N-butylimidazolium trifluoromethanesulfonate cuts out much of the volatility and waste. It handles reactions that push ordinary solvents too far, and it can even get reused, which appeals if you follow green chemistry principles. I remember seeing a colleague run palladium-catalyzed cross-coupling reactions with it—yield bumped up, and the product came out cleaner. A lower environmental toll isn’t just theory here.
Role in Batteries and Electrolytes
Battery teams, especially in research, keep experimenting with new electrolytes. This compound delivers wide electrochemical ranges, low vapor pressure, and strong ionic conductivity. That combination looks promising for lithium-ion batteries and supercapacitors. Electrolytes that stay stable during big temperature swings help electronics live longer. Safer batteries—ones that don’t leak or blow up—come closer to reality with ionic liquids like this.
Catalysis and Extraction
Industries rely on efficient catalysis for bulk chemicals, pharmaceuticals, and specialty products. Here, N-butylimidazolium trifluoromethanesulfonate unlocks new pathways. For example, in Suzuki reactions and alkylations, the solvent environment can make or break the process. I’ve seen research groups halve reaction steps by swapping traditional solvents for this ionic liquid. Time saved in process chemistry really adds up, especially where margins run tight. On top of that, this ionic liquid fits extraction processes where separating out target molecules from complex mixes gets challenging with water or oil alone. Those stubborn separations, including rare earth metals recovery, become more practical.
Environmental and Health Benefits
Switching to safer solvents pays off for both workers and the planet. Short-term, labs cut down on spills and toxic inhalation. Long-term, industrial-scale adoption puts less pollution into waterways and air. Regulatory pressure against volatile or carcinogenic chemicals only gets stricter. By moving to ionic liquids, companies can get ahead of bans and taxes—reducing future liability and clean-up costs. From a safety perspective, I’d much rather handle an ionic liquid over volatile acetone or hexane. A few spills on the bench mean less panic and fewer headaches later.
The Roadblocks and Way Forward
No magic bullet exists. The biggest challenges facing N-butylimidazolium trifluoromethanesulfonate are price and large-scale supply. Making these compounds takes specialized equipment and raw materials, so upfront costs can sting. Purity control also demands careful work, since even small contaminants may spoil reaction results or battery performance. The industry could push costs down by supporting more pilot projects, bulk synthesis, and robust recycling systems.
As labs and companies keep pressing for safer, greener, and more effective solutions, N-butylimidazolium trifluoromethanesulfonate will likely gain ground. Its role stretches from the bench up to pilot plants, offering a path away from old, hazardous chemistry toward something cleaner and more reliable.
Everyday Chemistry Meets Real-Life Responsibility
Chemicals like N-Butylimidazolium Trifluoromethanesulfonate show up in the lab because science keeps digging for new solutions. This ionic liquid stands out in research around catalysis, green chemistry, and electrochemical devices. As research pushes forward, careful handling becomes everyone’s responsibility on the workbench.
Storage: Keep It Dry, Keep It Cool
Most ionic liquids do best in tightly sealed containers, away from light and moisture. N-Butylimidazolium Trifluoromethanesulfonate falls under that same umbrella. It tends to soak up water from the air, which sometimes throws off reactions and makes measuring it a headache. Putting the bottle in a desiccator or dry box will cut down on moisture problems. Even inside a regular chemistry fridge, humidity lurks, so getting a good desiccant mix nearby pays off.
Cooler temperatures slow down most chemical changes. Leaving this compound at room temperature, away from sunlight and heat sources, leaves fewer chances for it to break down early. My old lab used amber glass for all light-sensitive or delicate chemicals. Simple choices like this can stretch shelf life, lower waste, and keep research budgets manageable.
Labeled, Locked, and Separate
Each container always got a label with date, concentration, and who opened it last. If a bottle looked odd, our safety manager checked for leaks or signs of pressure. These basic habits let everyone in the lab trust what they’re working with.
N-Butylimidazolium Trifluoromethanesulfonate should stay far from acids, strong bases, and oxidizers. None of these play nicely together, and all it takes is a rushed morning or a misread label to set off a dangerous mix. Supplying a dedicated shelf or bin, not next to solvents or high-risk chemicals, makes accidental contact less likely.
Protection and Good Airflow
Working with chemicals, you get used to safety goggles, gloves, and lab coats. What makes this compound stand out: it’s usually low in volatility, so fumes don’t jump out like with solvents. Still, I’ve watched colleagues react to the mild, sometimes harsh, smell after opening a container. Ventilated spaces, proper fume hoods, and frequent glove changes help guard everyone’s health.
Spills and Cleanups Happen
No matter how careful people are, spills show up. Our group posted cleanup steps at every workbench — lots of absorbent pads, neutralizing powder, and instructions for safe waste handling. A chemical like this shouldn’t go down any drain. Local waste collection or incineration matches shorter cleanup and less environmental risk.
Risks, Reporting, and Routine
Any unexpected reaction — like a slow pressure build, crystal formation, or color change — should trigger a report to the safety officer. Regular inventory checks help spot old or degraded material. Refresher safety training keeps everyone up to date, lowering odds of oversight when teams change or new projects start.
Better Habits Mean Safer Labs
Handling specialty chemicals draws a fine line between curiosity and caution. Small steps, clear habits, and shared experiences shape a safer, more productive lab brick by brick. In the case of N-Butylimidazolium Trifluoromethanesulfonate, respect for basic principles goes further than any warning label ever could.
Chemicals Like N-Butylimidazolium Trifluoromethanesulfonate: Practical Concerns Beyond the Lab
Many workers and researchers see long chemical names and instinctively worry, and for good reason. N-Butylimidazolium trifluoromethanesulfonate, with its mouthful of a name, comes up more often these days across green chemistry articles and industry research. It gets used as an “ionic liquid,” a modern solvent that stands out for generating less pollution than traditional organic options. Still, progress in science shouldn’t blind us to basic safety questions if it’s going to show up in more factories and research centers.
What We Know from Studies on Ionic Liquids
N-Butylimidazolium trifluoromethanesulfonate falls into a group of chemicals that drew attention for lower volatility and recycling potential. But many ionic liquids, including this one, don’t have decades of long-term health records. What we get instead are test results with cells, fish, and a few animal groups. These tests often compare toxicity to that of common lab solvents, many of which have well-known hazards.
Some ionic liquids get flagged for mutagenic or cytotoxic effects. N-Butylimidazolium trifluoromethanesulfonate appears less dangerous than older organic solvents, but it doesn’t get a free pass just because it feels “greener.” Environmental fate studies show ionic liquids tend to break down slowly in soil and water, which means any spills might linger, impacting aquatic life for more than just a few days. In fact, a 2015 study from the journal Chemosphere reported significant toxicity in aquatic species at concentrations much lower than those used in processing plants.
Human Hazards: What Industry Experience Teaches Us
Spending years around industrial and academic labs, you see patterns in how new chemicals get adopted. Rushed rollouts leave training gaps; workers often find themselves asking around—“Has anybody ever spilled this on their skin?” or “Can this stuff go down the drain?” Official Safety Data Sheets (SDS) for N-Butylimidazolium trifluoromethanesulfonate highlight skin and eye irritation as real risks. Inhalation risk isn’t as high as for more volatile chemicals, though that makes it easier to neglect until accidents happen.
Plenty of companies jump at “green” buzzwords, then lean on minimal personal protective gear, assuming lower risk. I saw a technician get a mild but concerning rash after handling a similar ionic liquid in a poorly ventilated room. Corporate assurance that a solvent is “not highly toxic” downplays the need for real precaution, even as skin and mild respiratory reactions continue to pop up in incident logs.
Sensible Protections and Oversight
Existing regulations haven’t caught up with emerging chemicals. Most safety calls rest with on-site supervisors and lab managers. Making assumptions about ionic liquids leads to careless disposal or poor spill procedures. The chemical’s persistence in water and soil means we should pressure manufacturers to treat spills and waste with extra care, much like more notorious solvents.
Unions and workplace safety organizations keep raising this point—don’t get lazy just because a chemical promises green benefits. Use gloves, store properly, avoid pouring any unused product down the drain, and demand more transparency from suppliers about toxicity testing.
Until we get long-term data, the safest road means sticking to protective measures and keeping disposal rigorous. A chemical with fewer known risks today isn’t always harmless in the real world, and learning that lesson too late can cost too much for both people and the environment.
Getting to Know the Compound
N-Butylimidazolium trifluoromethanesulfonate sounds quite a mouthful, yet it plays a real role in modern chemistry labs and makes a silent impact in greener industrial processes. The combination of an imidazolium ring with a trifluoromethanesulfonate anion produces a liquid salt that remains stable in demanding conditions. Thanks to this stability, researchers use it to replace more hazardous or volatile chemical solvents in organic synthesis, catalysis, and battery applications.
The Real Deal on Purity
Purity determines how well this compound performs. Many suppliers declare 98% or higher purity for technical and research-grade product. Some stretch attainment to 99%, which matters in the research world, particularly if your experiment’s success relies on zero unpredictable variables. I've witnessed results fall apart in the hands of an unnoticeable impurity—reaction yields drop, side-products creep in, and troubleshooting burns days you can't get back. If you plan to push the limits in electrochemistry or advanced synthesis, checking batch data for water content, halide levels, and organic residues counts for a lot more than a sticker on the bottle.
Impurities like water and halides often slip in during production or bottling. Water content should clock in below 0.2% by weight, as higher values start interfering, particularly in polar environments. Halide contamination sticks out as a troublemaker for those involved in catalysis or ionic conductivity measurements. Trace metals, left behind by production equipment or previous process steps, create an extra layer of unpredictability, so most reputable producers offer a certificate showing they stay below parts-per-million amounts. If not, it's worth asking.
Specification Sheet: Beyond the Headline
A solid specification digs deeper than just purity. Look for a breakdown showing exact levels of sodium, chloride, other common ions, as well as moisture and decomposition temperatures. In practice, melting points should hover above 60°C and thermal stability lasts up to 250°C—any lower, and decomposition may limit your applications. Density and viscosity usually feature on spec sheets too, as many applications in synthesis or extraction require consistent handling; higher viscosity can frustrate process control.
Color often offers a quick visual check. High-grade material shows up almost colorless to pale yellow. If a sample comes with obvious tint, chances are the manufacturer cut corners on the final wash or stored the bottle near sunlight. That extra color means more organic junk or by-products, which puts down the reliability of your process.
Solutions for Common Headaches
If you face quality hiccups, simple steps can help. I’ve run crude batches through activated charcoal, dried under vacuum over phosphorus pentoxide, and checked conductivity before getting results I’d trust with high-value syntheses. Investing in a reputable supplier—ideally one willing to share independent GC-MS or NMR analyses—pays off over time. Pushing suppliers for production and handling details, including lot traceability, narrows the risk of getting a bottle that upends a week’s worth of experiments.
Regulations keep evolving, and chemical makers tackle higher purity with better equipment and testing. Yet, real-life precision starts by requesting clear spec sheets and confirming numbers with your own bench tests. That’s the difference between chasing a paper guarantee and actually seeing results you can stand behind.
Looking at the Real Power of Ionic Liquids
Science tends to celebrate the headline stealers: big breakthroughs, shiny new devices, miracle drugs. Then ionic liquids come along—low-key, almost invisible in the news, yet pushing progress. N-Butylimidazolium trifluoromethanesulfonate, often glossed over in long lists of chemical compounds, belongs to this practical and versatile club. I’ve seen plenty of researchers chase big stories, but sometimes, the small details hidden in these liquids can change real-world processes.
Direct Benefits in Electrochemistry
The world keeps searching for better batteries and greener solvents. N-Butylimidazolium trifluoromethanesulfonate doesn’t easily evaporate and won’t catch fire as easily as organic solvents—so folks handling power storage devices can work with more confidence. In the lab, I have watched reactions run steadier with ionic liquids like this one, thanks to their wide electrochemical window and stability under electric currents. People building new batteries, sensors, or supercapacitors want something that handles both high and low voltages. Reports from teams across Asia and Europe show these ionic liquids supporting long-term cycling in devices, resisting the breakdown that hurts performance over time.
Supporting Progress in Catalysis
Catalysis stands at the core of industrial chemistry, from making fuels to recycling plastics. Traditional catalysts often struggle in volatile solvents, leading to safety headaches and pollution. Inviting N-Butylimidazolium trifluoromethanesulfonate as a solvent shifts this dynamic. The compound dissolves a wide range of chemicals, allowing catalysts to work in a safer, cleaner setting. This means fewer emissions into the air and fewer worries about flammable fumes. In practice, some labs have reported better control over reaction rates and fewer unwanted byproducts when bringing these “green” solvents into play.
Addressing the Downsides
Strengths aside, this isn’t an all-purpose fix. Ionic liquids like N-Butylimidazolium trifluoromethanesulfonate cost more than regular solvents, so only processes needing their special qualities justify the price. Not every system wants a triflate counterion, either; sometimes it interferes with catalysts. Some researchers point to uncertainties about how best to recycle or dispose of these compounds, since waste rules still catch up slowly to new chemistry.
Steps Toward Broader Adoption
It’s not just about swapping in a new liquid and walking away. Industries aiming to cut pollution or run safer labs need training and sometimes new equipment. Regulators want proof that ionic liquids like this one won’t harm workers or water sources. Funding more research into recovery and purification methods can help bring costs down and relieve pressure on supply chains. Reuse, rather than dumping, matters both economically and for public trust. Sharing lessons between academic labs and manufacturers leads to faster solutions and real technical progress.
What the Future Could Hold
N-Butylimidazolium trifluoromethanesulfonate shows up where chemistry needs stability, safety, and new reaction pathways. Battery designers and industrial chemists who tap into its properties often see smoother performance and cleaner work. My own experience working with ionic liquids has taught me the importance of weighing up cost, benefits, and long-term safety. Trust in a compound builds with every successful experiment, making it easier for others to follow. Sometimes change starts with swapping out one stubborn solvent, opening doors to greener, safer, and more reliable processes in the lab and beyond.