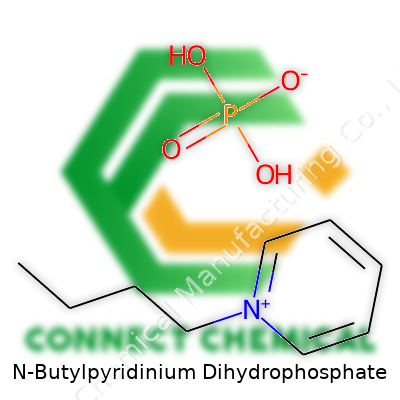
N-Butylpyridinium Dihydrophosphate: A Closer Look from Lab Bench to Practical Use
Historical Development
Back in the early 2000s, a handful of chemists started to rethink the limitations of traditional solvents. The quest for better and safer reaction media led researchers to focus on ionic liquids, and among them, N-Butylpyridinium Dihydrophosphate began to get some real attention. I remember working in an academic lab where green chemistry became a buzzword, and every week someone tried out a new ionic liquid hoping to stumble on something with less volatility, lower toxicity, and maybe even a recyclability angle. The promise here was to swap out the usual harsh acids or organic solvents for something more forgiving, and this compound looked like a real contender. Researchers kept returning to its stability and interesting blend of organic and inorganic components—pyridinium from the aromatic world, butyl groups lending hydrophobic flavor, and a phosphate bringing the ionic heft.
Product Overview
This compound lines up as a clear liquid at room temperature, packing a punch with its ionic structure. Most suppliers deliver it in amber vials to minimize light exposure, and for good reason—light can slowly nudge the pyridinium ring down a photo-chemical pathway that messes with purity. What stands out is a scent that reminds some of faint esters, not the aggressive tang found in typical ammonium salts. Applications require high purity material, so vendors print the batch number and purity percentage (usually above 98%) right on the label. Focusing on quality control, labs tend to verify water content regularly—less than 0.5% by Karl Fischer titration helps maintain repeatability in sensitive syntheses. In my own bench work, a lot depends on confidence in these numbers, and suppliers who skimp on batch analysis often get left out of grant budgets.
Physical & Chemical Properties
N-Butylpyridinium Dihydrophosphate appears as a viscous liquid. It clocks in with a melting point well below room temperature—often in the range of -40°C to -20°C. That low point matters during winter shipments; cold chain logistics sometimes falter, leaving users with thickened, sluggish samples. It carries a density of around 1.15 g/mL, meaning pipetting calls for a different hand-feel than water or acetonitrile. The ionic character leads to negligible vapor pressure. You’ll never see clouds of this stuff drifting off the bench during volatilization. From a chemistry point of view, the molecule blends a basic aromatic ring with a soft alkyl chain, anchored by a dihydrophosphate group that can shake hands with water or polar organic solvents. This unique make-up supports remarkable solubility—eagerly mixing with both protic and aprotic media, which pays off during multi-step syntheses or catalysis. The pH sits slightly acidic in water, which impacts certain reaction mechanisms but also helps in stabilization.
Technical Specifications & Labeling
Open a certified bottle and you’ll see the labels mention the compound’s systematic name, molecular formula C9H16NO4P, molecular weight of roughly 233 g/mol, and CAS registry number—details that stay essential for chemical tracking and regulatory compliance at any scale of work. SDS and CoA sheets accompany most shipments; these aren’t paperwork for the sake of it. They lay out handling and disposal guidelines aligning with university and industrial protocols. Shelf life hovers around two years if stored in cool, dry conditions, usually below 25°C. High humidity leads to slow hydrolysis, so climate control becomes key if you’re using open vials over months. Labels also display potential hazard statements: hazard pictograms often show up, especially in European labs where CLP regulations require explicit warning of skin and eye irritation risks.
Preparation Method
Synthesis of N-Butylpyridinium Dihydrophosphate tracks back to classic quaternization followed by anion exchange. Starting with pyridine, an alkylation reaction using 1-bromobutane delivers the butylpyridinium bromide salt. This stage often involves phase transfer catalysis or simply excess reactant to push yield upward. After purification, usually via repeated washing and evaporation, a double exchange with silver dihydrophosphate in water or methanol replaces the bromide with the coveted dihydrophosphate anion. The silver halide byproduct gets filtered off. For academic operators, scaling from milligram up to kilogram quantities depends on careful control of water content and temperature: excess moisture introduces unwanted side reactions, while high heat can damage the aromatic ring structure. I’ve watched groups battle with this, finally investing in higher-grade solvents and gloveboxes to hit high purity batches.
Chemical Reactions & Modifications
Chemists find this compound useful both as a medium and as a participant. In organocatalysis, it stabilizes transition states while occasionally activating key substrates via hydrogen bonding or ionic interactions. Because of its dual affinity for hydrophobic and hydrophilic partners, reactions involving nucleophilic substitutions or cycloadditions sometimes yield better selectivity here than with classic solvents. One standout feature: the aromatic ring offers points for further functionalization. I’ve seen papers where the butyl side chain is swapped for longer or branched alkyl groups, fine-tuning solubility or thermal stability. N-Butylpyridinium Dihydrophosphate also participates in acid-catalyzed esterifications and transesterifications, thanks to its ability to donate or shuttle protons through the phosphate group. Some research groups play with embedding it in polymer networks, aiming to make novel membrane materials or sensors, though that work sits more in the exploratory zone.
Synonyms & Product Names
Across the literature and chemical supply catalogs, other names sometimes pop up, which leads to confusion among newcomers. On top of the full IUPAC title, you’ll encounter n-butylpyridinium phosphate, or 1-butylpyridinium dihydrogen phosphate, and occasionally, the shorthand [BuPy][DHP] in journal articles. Careful product selection matters because similar-sounding names, like butylpyridinium tetrafluoroborate, possess very different handling risks and reactivity. For tracking, a quick cross-check using CAS number helps avoid misordering and keeps inventory managers from pulling the wrong bottle during audits.
Safety & Operational Standards
Handling N-Butylpyridinium Dihydrophosphate requires knowledge of its potential to cause irritation on contact—a trait shared by other ionic liquids. No need for full hazmat suits, but both lab coats and nitrile gloves stay mandatory, and eye protection proves non-negotiable once you’ve witnessed a splash accident. Users notice skin drying or mild redness with careless handling. Ventilated hoods help because trace vaporization contributes to cumulative exposure, especially during long synthetic runs. Disposal must avoid aquatic environments, since ionic liquids persist in water and need separation in hazardous waste containers—most university protocols include weekly pickups to minimize risk buildup. In industrial settings, standard operating procedures call for emergency eyewash stations and material spill kits close by. I’ve found that routine training cuts down on accidents and reduces downtime from cleanup.
Application Area
Labs ranging from academia to pilot-scale industry turn to N-Butylpyridinium Dihydrophosphate for extracting delicate biomolecules or running selective clean fuel transformations. Its stability and ionic nature make it a favorite in biomass conversions—taking cellulose or lignin apart with fewer side-reactions than strong mineral acids. Electrochemists research it as an electrolyte in next-generation batteries, exploiting low volatility and high ionic conductivity to push energy density and safety boundaries. I’ve advised startups using similar compounds for dye-sensitized solar cells, drawn to their ability to keep dye molecules dispersed and happy in thin film assemblies. Catalysis researchers favor this liquid for Brønsted acid-sensitive reactions that demand a gentle environment. Real-world feedback suggests the compound’s biggest fans work on problems where minimizing waste, managing selective transformations, or improving process yields actually move the bottom line.
Research & Development
Research groups in both university and commercial settings continue to dive deep into this chemical’s performance spectrum. Current work revolves around custom-tuning alkyl chains, adjusting hydrophobicity, and adding metal or biomolecule functionality to the anion. These modifications aim to create more specialized solvents for pharmaceuticals or for recycling valuable transition metals from spent catalysts. Academics, including grad students on tight budgets, keep tracking green chemistry metrics—E-factors, recyclability, and energy inputs—testing whether this liquid holds up in real continuous processes. A wave of collaborations with engineering departments explores how these ionic liquids handle in larger reactors and purification schemes, where mass transport and phase separation matter just as much as a good NMR spectrum. The trickiest part of the work keeps circling back to balancing performance with readiness for regulatory clearance in food, pharma, or energy applications, especially under Europe’s tightening REACH guidelines.
Toxicity Research
Toxicologists and environmental chemists raise ongoing questions about persistence and chronic exposure. Compared with traditional organic solvents, acute toxicity in mammals looks modest, based on existing LD50 data in rodent studies, but environmental fate remains less charted. Several studies report limited biodegradability, raising red flags for aquatic toxicity and bioaccumulation. Wastewater engineers run pilot trials to test breakdown methods, such as advanced oxidation or bio-filtration, with mixed results. Experience suggests that, while acute risks to operators stay mild, even small releases over years could generate measurable signals in effluent streams. Until long-term testing catches up, prudent practice includes closed-loop handling and strong spill remediation plans. Institutions with green chemistry mandates sometimes limit use until more data comes in—and researchers have learned to address these gaps up front in funding applications.
Future Prospects
Looking ahead, N-Butylpyridinium Dihydrophosphate’s utility in sustainable process chemistry grows as researchers chase solvent systems that boost yields and cut overall hazards. If biotechnologists further unlock routes to synthesize ionic liquids from renewable feedstocks, the market could shift deeper into green territory. Energy storage applications entice with promises of safer and more powerful batteries or supercapacitors—a direct response to questions about lithium-ion volatility and lifetime. Many see a new competitive landscape where research pivots to products with both performance and regulatory legibility. From my years troubleshooting process rollouts, market expansion hinges on the compound’s lifecycle data, reliability in real-world conditions, and manufacturers’ willingness to publish operational best practices. As data fills in, the hope remains for a material that bridges the gap between chemical innovation and practical, scalable utility.
A Closer Look at Its Most Important Roles
N-Butylpyridinium dihydrophosphate isn’t a name you hear during dinner conversations. Still, in labs and processing plants, this ionic liquid brings fresh solutions to tricky problems. My own work with new electrolytes pulled me into research on this compound years ago, and I quickly saw why chemists keep it close at hand. As a functional material, it carves its own spot in different sectors.
Electrochemistry: Making Batteries Safer and More Stable
Stability tops the list for battery components. Traditional solvents, which often evaporate or catch fire, fall short in safety tests. N-Butylpyridinium dihydrophosphate acts as a stable ionic liquid and can replace those risky solvents in electrolytes, especially in lithium-ion and supercapacitor technology. The phosphate part boosts thermal stability, letting cells run at high temperature without breaking down. This means batteries built with this liquid resist swelling, leaking, and short-circuiting, which is a big step up for electric vehicle and grid storage safety.
Green Chemistry and Catalysis: Cleaner Solutions
With old-style organic solvents, chemists often end up with a lot of waste. N-Butylpyridinium dihydrophosphate, on the other hand, doesn’t evaporate as quickly. In my own trials, replacing common solvents with this ionic liquid shrank hazardous waste after catalyst recovery, and clean-up felt easier on the lab environment and my conscience. The compound works as both solvent and catalyst in several organic reactions, including esterification and alkylation, showing solid activity and selectivity. This saves energy by allowing lower reaction temperatures and cuts down post-reaction processing.
Biochemical Research: A Friend to Enzymes
Researchers often look for liquids that won’t wreck delicate enzymes. N-Butylpyridinium dihydrophosphate interacts gently with proteins and sometimes even boosts their stability compared to water-based mixtures or volatile organics. Experiments using enzymes for pharmaceutical synthesis or diagnostics benefit from this; the results stay consistent, and enzyme life extends. Some published reports point to smoother handling in enzyme immobilization and bioconversion processes. From personal lab runs, enzymes held their shape longer, meaning less downtime chasing replacements.
Extraction and Separation: Greener Industrial Processing
Extracting valuable chemicals from complex mixtures uses tons of solvents, most of which never get recovered. With this ionic liquid, the separation process becomes both cleaner and more efficient. In the materials recovery field, N-Butylpyridinium dihydrophosphate has helped remove heavy metals and rare elements from water streams and industrial waste. I’ve observed higher selectivity for targeted ions, cutting back on byproducts. That leads to better recycling of metals and smaller loads headed for hazardous waste treatment.
Pushing for Safer, Smarter Chemistry
The search for less toxic, more sustainable chemicals never really stops. N-Butylpyridinium dihydrophosphate doesn’t solve every challenge in industry or the lab, but seeing the range of jobs it takes on — from keeping batteries safer to making chemical processes greener — highlights why it attracts so much attention. The compound stands out where safety, stability, and environmental impact all matter. Teams in battery research, catalysis, green manufacturing, and biotech are using this ionic liquid to replace old ways with options that produce fewer risks and better results.
The Bones of the Molecule
N-Butylpyridinium dihydrophosphate isn’t a household name, but in the world of chemistry, its structure stands out. At its core, the molecule features a pyridine ring—a six-membered aromatic structure with one nitrogen atom tucked in with five carbons. Swap out the usual hydrogen on nitrogen for a butyl group, and there you have the N-butylpyridinium cation. Slotted next to this is the dihydrophosphate anion, a phosphate group carrying two hydrogens, which can make it behave a little differently when mixed with water or other solvents.
Drawing the Structural Map
Chemists look at N-butylpyridinium dihydrophosphate and see a series of connections: the butyl chain (C4H9) dangles off the nitrogen on the pyridine ring (C5H5N). Together, this forms the cation: C9H14N+.The anion, dihydrophosphate, looks like H2PO4–. When balancing the charges, these come together as an ionic pair.Bring these parts together, and the typical molecular formula shows up as C9H14N·H2PO4.
Why It Matters in Labs and Industry
N-butylpyridinium dihydrophosphate works as more than just another chemical on a shelf. Its ionic nature means it fits well as an ionic liquid—a special group of salts that stay liquid at room temperature or just above it. Scientists lean on these liquids for their low volatility and ability to dissolve a wide range of materials. Safety and environmental benefits come into play since they don’t usually give off harsh fumes or catch fire as easily as common organic solvents.
The structure opens the door to fine-tuned solvents. By adjusting the chain on the pyridinium ring, labs can tweak solubility and viscosity. This kind of control matters for green chemistry projects, drug formulations, and complex separations in research and manufacturing. In my own work handling small-scale syntheses, I search for solvents that won’t ruin sensitive compounds. Ionic liquids like N-butylpyridinium dihydrophosphate offer that gentle touch while carrying enough strength to help reactions move along.
Sustainability and Safety
As more companies chase greener alternatives to old-school solvents, structures like this start turning heads. Traditional solvents—toluene, benzene, even ethanol—can create messes that stay in water or soil for years. Ionic liquids, shaped by molecules like this, help cut down on those risks. Low vapor pressure means lower risk for explosions or chronic exposure, a huge plus for folks who spend day after day elbow-deep in glassware and vials.
Challenges and Future Possibilities
One trip through the literature makes it clear: even if ionic liquids look promising, they bring quirks that demand respect. Some break down under heat, others might not work as well as hoped in living systems. Researchers now look for ways to make these liquids with fewer toxic byproducts and improved biodegradability. Responsible disposal and recycling of these substances form the backbone of safe lab practices and industrial management.
Collaboration between lab scientists, environmental engineers, and industrial chemists will drive progress. Tracking the fate of N-butylpyridinium dihydrophosphate and similar molecules, asking tough questions about toxicity, and designing future versions with the planet in mind, will sort out whether these smart solvents can step up and push chemistry in a cleaner, safer direction.
Why Chemical Safety Rules Hit Home
I’ve worked in labs where every drawer and bottle matters. Management likes to stress that chemicals demand attention, but people don’t always see why until someone skips a step and the results get messy. N-Butylpyridinium Dihydrophosphate doesn’t make headlines, but if you ignore the protocols with this compound, people and property pay the price. Chemistry doesn’t forgive shortcuts.
What Makes N-Butylpyridinium Dihydrophosphate Different?
This isn’t a household name. It’s an ionic liquid, formed from mixing a pyridinium derivative with dihydrophosphate. Ionic liquids stand apart: they rarely evaporate, but that doesn’t mean they won’t react. N-Butylpyridinium Dihydrophosphate draws water from the air. Left open, it becomes sludgy and breaks down — and nobody wants to handle a degraded chemical. Moisture transforms both the liquid and the risks.
Simple Steps to Safer Storage
Keep the container tightly closed. Even a quick exposure to air can start a reaction or draw water into the bottle. Don’t stack containers near sunlight or heat. This chemical won’t catch fire easily, but it does decompose when temperatures rise. Store it where the thermometer stays cool, below 25°C, and humidity doesn’t creep in. Chemical-grade refrigerators work best if there is any doubt.
Choose the right kind of container. Glass and certain plastics hold up well, but avoid metals that could corrode or react with phosphates and ionic liquids. Double-check seals and lids for leaks before every use. I’ve seen older containers with loose caps that turn storage cabinets into unsafe zones.
Clean Handling Protects More than the Chemical
Use gloves and goggles — not because rules say so, but because one splash can sting and cause irritation. The compound isn’t acutely toxic, but long-term exposure to many pyridinium derivatives hasn’t been fully studied. Skin and eye contact bring their own issues. Keep pipettes, syringes, and labware dedicated for ionic liquids, and cleanse everything right after use. Residue left behind increases cross-contamination risk, and that’s a headache labs should avoid.
Move the bottle slowly, and never pour over open workspaces. Spills roll fast and soak into wood, paper, or porous surfaces. I once watched a spill eat through paper notes, leaving a sticky mess nobody wanted to clean. Absorb with sand or vermiculite, not your regular mop. Dispose of every spill container according to chemical waste rules, not in the standard trash.
Building a Culture of Respect
Every new chemist faces the temptation to cut corners. With N-Butylpyridinium Dihydrophosphate, routines matter. Safety Data Sheets outline hazards, but coworkers and supervisors shape habits. Share tips and observations, don’t mock caution, and call a timeout when something feels off.
Protecting people and chemicals comes down to shared vigilance. Follow rules today and avoid regret tomorrow; that’s a lesson I learned the hard way, and nobody forgets once it happens. N-Butylpyridinium Dihydrophosphate won’t forgive any lapse, so respect the substance and teach others to do the same.
Understanding the Chemical
Chemists searching for greener solvents have turned to ionic liquids like N-Butylpyridinium Dihydrophosphate, drawn by their promise. These liquid salts sit at the crossroads of industrial interest and environmental concern, especially as their use widens in labs and manufacturing. Makers see them as useful because they resist evaporation, and some believe that their “green” label means less danger to people and the earth.
Human Health Risks
Dealing with chemicals day in and out, anyone in a synthesis lab knows that safety isn’t just about fumes or flammability. Ionic liquids often avoid the hazards of volatile solvents, but they can trade one risk for another, especially by being persistent or irritating. In a 2011 study, researchers found certain pyridinium-based ionic liquids—including those with butyl groups—caused skin and eye irritation in test models. Symptoms included redness and swelling after direct contact.
Workers handling this material without gloves or goggles could face inflammation if the liquid spills. Inhalation doesn’t come up as a big issue for N-Butylpyridinium Dihydrophosphate, but accidental splashes to the eyes or onto skin remain a daily risk. As with so many ionic liquids, there's a knowledge gap. Publicly available research only paints part of the picture—no long-term chronic effect studies exist for this exact compound in humans. Based on related chemicals, repeated or high exposure could mess with the skin’s barrier and even impact cells, but nobody has tracked enough people over time to say for sure.
Environmental Perspectives
Before ionic liquids gained their “benign” reputation, people assumed their low vapor pressures eliminated most risk. That’s a false sense of security. N-Butylpyridinium Dihydrophosphate resists breakdown, so after disposal it can stick around in waterways and soils. Studies from the 2010s flagged several ionic liquids as moderate to high in aquatic toxicity—even small doses harmed algae, water fleas, and fish embryos.
Pyridinium-based versions have shown persistent toxicity both in freshwater and marine ecosystems. Some of these salts affect enzymes in aquatic species, messing up normal growth and development. That’s a far cry from harmless. If the liquid enters a sewer or landfill, it doesn’t just vanish. Accumulation can hurt local ecology, especially when conventional water treatment plants don’t remove such compounds. Everyday folks rarely realize each solvent, even a “green” one, has a downstream effect.
Reducing the Hazards
Better training and workplace procedures make a difference for anyone handling the material. Chemical-resistant gloves and splash protection help protect skin and eyes. Ending the “green” myth involves clear labels and honest hazard statements. Scientists need to design disposal routes for used liquids, not just dump them. Treatment or incineration under controlled conditions keeps waterways cleaner.
Regulators and manufacturers should push for more testing—including chronic toxicity, biodegradation rates, and ecosystem effects—before adopting new variants. University labs, where many of these liquids are pioneered, can set the standard by sharing safety results and disposal methods transparently. Honest, thorough communication keeps both people and the planet safer than recycled claims about “greener alternatives.”
What Chemists Can Expect from Modern Suppliers
Anyone working with ionic liquids like N-Butylpyridinium Dihydrophosphate needs to look twice at both the purity and packaging offered. Both aspects shape results in a real lab environment, sometimes more than the price tag. Sooner or later, inconsistent product quality causes headaches, wasting time and cost. From a practical lens, this compound usually reaches labs at purities above 98%, with premium suppliers pushing that figure closer to 99%. That difference may sound tiny, but trace impurities can steer a reaction down the wrong path.
The Impact of Purity on Research and Industry
Researchers use N-Butylpyridinium Dihydrophosphate in tasks that never leave much room for error—think catalysis, electrochemical projects, or synthesis involving sensitive starting materials. Just one percent of unknowns or moisture can chew up entire batches, especially with moisture-sensitive intermediates or ionic conductivity studies. Labs aiming for publishable data—or scaling up—quickly realize the cost of cutting corners on purity. There is a ripple effect through downstream experiments. From my own time assisting in a university lab, we paid extra for higher grade and skipped plenty of troubleshooting that cheaper lots brought to the table.
Packaging Sizes: Why Small and Large Both Matter
The days of one-size-fits-all containers have mostly passed. Common packaging sizes for N-Butylpyridinium Dihydrophosphate now include as little as 5 grams for trials, up to kilo-sized bottles or drums for scale-up or pilot runs. A small lab usually sticks to 10 or 25-gram bottles. These quantities minimize waste and cut down on contamination risks from multiple openings. Shops and procurement officers for larger facilities often go for 100-gram, 250-gram, or even 1-kilo units. Flexible sizing keeps shelf stock fresh and keeps storage hazards low.
Quality Assurance Beyond the Label
Buying a chemical based only on purity on the label rarely delivers the peace of mind that long-term projects need. Reliable suppliers back their claims with COAs—Certificates of Analysis—that lay out real numbers on water content, main impurity profiles, and storage dates. Nobody likes a surprise after months of careful planning. There’s trust in simply seeing exact batch numbers and plenty of contact with customer support for technical details. Well-run companies also offer tamper-evident seals and clearly print expiration dates, which matter just as much as the chemical itself.
Solutions to Sourcing Issues
Researchers and industry buyers often benefit from partnering directly with suppliers who know their own supply chains. Miscommunication or mishandling can kick up when third parties get in the middle. Ordering directly, or at least requesting detailed batch data, helps avoid delays from ingredients going out of spec. There’s also value in storing quantities in cool, dry spaces with good labeling. I’ve seen poorly stored ionic liquids degrade before use; maintaining stable inventory beats reacting to last-minute surprises.
Looking Ahead with Trustworthy Sourcing
N-Butylpyridinium Dihydrophosphate plays its part in everything from greener chemistry to new energy storage projects. Pushing the boundaries in the lab or at pilot scale sometimes comes down to having the right purity and easy access to the right container size. In the end, putting effort into sourcing high-quality material pays back through smooth experiments and results that stand up to scrutiny.