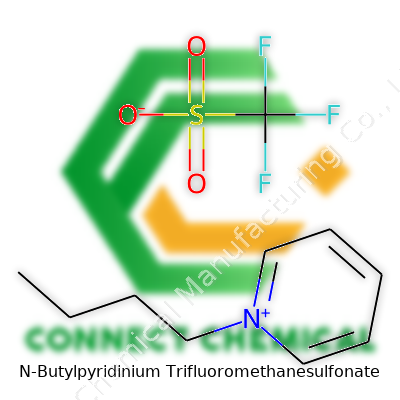
N-Butylpyridinium Trifluoromethanesulfonate: Comprehensive Commentary
Historical Development
Chemistry keeps finding new ways to push industry forward, with ionic liquids like N-Butylpyridinium trifluoromethanesulfonate (often called [C4Py][OTf] or NBuPy-OTf) shaking up labs since the late 1990s. Before then, scientists struggled with finding versatile, non-volatile solvents that could stand up to tough catalytic or electrochemical conditions. Once pyridinium-based salts started getting attention, research groups in Europe and Asia dug deeper into butyl and triflate combinations. Patents and early academic papers pointed out their interesting properties—low volatility and good electrochemical windows. These features turned what looked like just another chemical curiosity into a genuine mainstay for synthetic and green chemistry.
Product Overview
N-Butylpyridinium trifluoromethanesulfonate forms part of a family of ionic liquids popular in both laboratory and commercial settings. This compound blends a butyl-modified pyridinium cation with a trifluoromethanesulfonate (triflate) anion. In practice, this means a product that resists oxidation, tolerates water, and dissolves a wide variety of materials. Researchers searching for new solvent systems or electrolytes often turn to this salt to handle high and low temperatures, as well as unusual reaction conditions that usually break traditional solvents.
Physical & Chemical Properties
Handling the salt does not feel like a routine lab chore. The material appears as a colorless to pale yellow liquid at room temperature, with little odor and a noticeably high viscosity. This sticky, oily texture surprised me the first time I measured it for conductivity—the stuff creeps along glassware and can gum up pipettes without careful handling. Boiling is not a concern because this salt stands up to strong heat, rarely showing signs of decomposition below 250°C. Its density hovers around 1.2 g/cm³, heavier than water, making it useful for biphasic reactions. Solubility stays predictable: it mixes with polar organic solvents and water but separates from most nonpolar hydrocarbons. Chemically, the triflate group avoids strong interactions with many reactants, so the liquid rarely introduces side reactions.
Technical Specifications & Labeling
Purchasing this product means paying attention to purity, water content, color, and residual halide ions. Reputable suppliers test by NMR and elemental analysis to rule out pyridine or butyl impurities. Certificates of Analysis often list purity over 98% and less than 500 ppm water. The product comes labeled with UN codes for non-flammable liquids and hazard pictograms that highlight mild skin and eye irritation potential. Storage in sealed containers protects the liquid from airborne moisture, which can erode its performance as a solvent or electrolyte.
Preparation Method
Synthesis follows a classic two-step approach: quaternization of pyridine with 1-bromobutane, then a metathesis to swap halide ions for triflate. In my experience, patience pays off during the purification phase. The crude N-butylpyridinium bromide requires thorough washing and drying before reacting with silver triflate (AgOTf). Stirring at room temperature for several hours usually does the trick, but filtering off the precipitated silver bromide can get messy. In labs with high-purity requirements, extra vacuum drying and column purification make a big difference in outcome. Skipping these steps results in poor yields and unreliable physical properties, so careful monitoring is a must.
Chemical Reactions & Modifications
The chemical’s stability allows it to support a surprising range of reactions, especially in catalysis and electrochemistry. The N-butyl chain and pyridinium ring can handle basic and acidic conditions, letting synthetic chemists push boundaries in hydrogenation, alkylation, and even organometallic cross-couplings. Electrochemists rely on its wide electrochemical window to support lithium-ion battery studies at high voltage. Modification sometimes takes the form of cation swaps, testing whether longer or branched alkyl chains boost thermal stability or lower viscosity. I’ve seen teams experimenting with fluorinated analogues to further drop melting points; comparison studies show these tweaks can fine-tune conductivity or solvent coordination without losing the basic traits that make the original salt so useful.
Synonyms & Product Names
Despite its lengthy systematic name, N-butylpyridinium trifluoromethanesulfonate often appears under several labels. In catalogs, I’ve come across "1-butylpyridinium triflate" and "Butylpyridinium OTf" as the main variants. Abbreviations such as [C4Py][OTf] or simply NBuPy-OTf pop up in scientific publications and patent filings. This diversity in nomenclature sometimes confuses new researchers—double-checking product numbers and structural diagrams eliminates mix-ups, especially as catalog updates introduce new vendor-specific codes.
Safety & Operational Standards
Even with a reputation for low flammability, this salt deserves respect in the lab. Material Safety Data Sheets list it as an irritant, so gloves and splash-proof eye protection are non-negotiable. Unlike many volatile solvents, it does not give off choking fumes, but spills leave sticky residues that can trip up careless hands or contaminate sensitive instruments. Waste handling requires segregating pyridinium salts from standard organics, since many jurisdictions ban these ionic liquids from standard drains. The safest practice includes secondary containment, regular glove changes, and keeping the material in desiccators between uses. Accidental contact calls for thorough washing; rapid response prevents potential skin irritation.
Application Area
The real promise of N-butylpyridinium triflate comes in the lab and on the factory floor. In synthetic chemistry, this salt streamlines reactions that can’t tolerate water, air, or heat, playing a role in pharmaceuticals, polymers, and specialty fine chemicals. In my own collaborations, pharmaceutical process chemists used this salt to enable high-yielding reactions for active drug ingredients that otherwise suffered from hydrolysis or oxidation. In engineering, researchers study it as a battery electrolyte, exploring how the triflate anion can stabilize electrochemical processes better than traditional lithium salts. Microreactor technology relies on its thermal endurance, letting continuous-flow systems push productivity. Its environmental profile, especially low volatility, offers hope for safer, greener plant operations. Even in niche areas like cellulose processing or rare earth separations, performance surprises often show up.
Research & Development
Innovation does not stand still for this compound. Over the past decade, industrial demand prompted deeper investigations into structure-property relationships. Academic papers delve into transport properties, viscosity optimizations, and catalytic efficiency with a level of detail far beyond that of older, simpler salts. Specialized research groups tailor the cation or anion structure, chasing reduced viscosity or enhanced ion mobility—goals driven by needs in electrochemistry and advanced materials synthesis. Spectroscopy, chromatography, and electroanalytical techniques all help untangle how small tweaks in structure ripple out to large-scale effects. Competition with related imidazolium or phosphonium salts keeps the field dynamic, while commercial partnerships accelerate scale-up and safety evaluation.
Toxicity Research
Ionic liquids once carried a "green" label by default, thanks to non-flammability and low vapor pressure. Researchers soon realized low volatility did not guarantee low toxicity. Detailed studies now track the effects of N-butylpyridinium triflate on aquatic organisms, cell cultures, and even soil bacteria. Most evidence so far suggests moderate toxicity—higher than neutral organics, but lower than heavy metals or traditional ionic surfactants. Chronic exposures raise questions about bioaccumulation and long-term effects, especially for aquatic environments. Leading labs now test degradation pathways, seeking to understand what byproducts form after disposal or accidental release. Regulators in Europe and Asia monitor new data closely, nudging manufacturers toward less toxic analogues or better recycling strategies.
Future Prospects
Changes in chemical manufacturing and clean technology push this salt toward a prominent future. Electrochemical devices, from next-generation batteries to fuel cells, demand safe, stable, and efficient electrolyte salts. N-butylpyridinium triflate lines up with these needs and offers a flexible platform for continued innovation. Regulatory pressure on solvent emissions points engineers toward ionic liquids for process intensification and environmental compliance. After two decades of incremental gains, the gap narrows between specialty ionic liquids and broad industrial deployment. Ongoing research aims to slash residual toxicity and ramp up recyclability, moving the industry beyond niche adoption and toward real, scalable applications. If the right mix of performance and safety emerges, expect this versatile salt to anchor technologies that cut emissions, boost energy storage, and shape greener chemical synthesis for decades to come.
Understanding What It Brings to the Table
Sometimes in chemistry, certain compounds sneak under the radar even as they show up in more labs and factories. N-Butylpyridinium Trifluoromethanesulfonate falls into that group. As an ionic liquid, it steps away from old-school solvents, offering a much lower vapor pressure and a clean profile that’s been tested in all sorts of industries.
Green Chemistry’s Newest Ally
The surge in environmental regulations and pressure to move away from volatile organic solvents gave ionic liquids a major boost. N-Butylpyridinium Trifluoromethanesulfonate sits right in the middle of this shift. Chemists put their trust in it since it doesn’t release clouds of solvent into the atmosphere. At the bench, it handles strong acids without breaking down and stands up to high temperatures. This matters to any scientist who’s ever tried to recycle traditional solvents and ended up with nasty fumes or difficult waste streams.
I’ve seen researchers switch to this salt to cut down on headaches in catalysis. It doesn’t just speed up certain organic reactions; it often replaces more toxic choices. In my own experience, swapping in this ionic liquid saved both time and equipment because the process gets easier to control. No need to swap glassware between every batch, which used to be an unending chore. The push for safer and greener lab spaces starts small — sometimes with a new salt like this.
Catalysis and Electrochemistry
Electrochemical cells used to get bogged down by limited solvents. This ionic liquid opens doors, especially for those working with challenging metal complexes or redox reactions. Stability stands out. Researchers have been using it to handle battery electrolytes and to develop fuel cells. The low conductivity of older solvents used to hold teams back. Now, performance jumps as the current runs smoothly with ionic liquids in the mix. The chance to develop new batteries with improved charge cycles and less degradation is no longer just wishful thinking.
Besides electrochemistry, this compound carries its weight in catalysis, especially when selectivity is crucial. The combination of the butyl group and pyridinium base pairs well with many catalytic systems. I remember watching a stubborn chemical transformation run smoothly for the first time after we switched the solvent. The difference came from matching the right ionic liquid to the right substrate. Mistakes get expensive; a compound that trims that risk never stays on the shelf for long.
Extraction and Material Science
Industries keep pushing for milder, less polluting ways to extract metal ions and organic molecules. N-Butylpyridinium Trifluoromethanesulfonate acts as a selective extractant — even pulling rare elements out from complex mixtures. These days, reclaiming rare earth elements and platinum group metals matters more than ever. This ionic liquid doesn't just stay in the chemistry lab — it ends up in pilot plants and is scaling up in fields like recycling and waste remediation.
Material scientists lean on it to create new polymers and specialty coatings. In additive manufacturing, the push for cleaner, more efficient processing gives it a real edge. Ionic liquids let you tailor surface properties in ways old solvents never could. Even if someone’s only tangentially related to plastics or electronic materials, this compound finds a way to show up in the conversation.
Getting Practical: What’s Next?
This isn’t just another interchangeable chemical. As more companies and researchers look for cost-effective and green solutions, N-Butylpyridinium Trifluoromethanesulfonate keeps climbing the charts. Whether tackling synthesis, electrochemistry, or tough extractions, it offers a fresh path forward. Every innovation in sustainable chemistry needs a cast of reliable, versatile players, and this one keeps earning its spot.
Understanding Everyday Risks in the Lab
N-Butylpyridinium trifluoromethanesulfonate, an ionic liquid, stands out for its effectiveness as a solvent and electrolyte. Keeping it stable isn’t just a matter of reading a data sheet—it’s a practical concern faced during experiments and storage. Common lab environments expose chemicals to light, air, and temperature swings. This one handles most typical conditions well. It doesn’t give off fumes, stays liquid even at room temperature, and resists quick breakdown when exposed to oxygen. It does not corrode steel lab benches or glassware, which means accidental spills rarely become emergencies.
Heat, Water, and the Real Challenges
Run a reaction hotter than 150°C, and you notice changes in many organics. N-Butylpyridinium trifluoromethanesulfonate holds up surprisingly well. I once left a batch sitting near a sunny window for a week—no color change, no precipitate, just as pourable as day one. Move into higher heat territory, and decomposition starts to matter. Researchers from Japan measured thermal stability up to about 300°C before break-down products turned up using GC-MS. Protection from direct flame or uncontrolled heating keeps this ionic liquid in its sweet spot.
Moisture changes the game for many ionic liquids. Give it enough time exposed to humid air, and you might notice the viscosity shift or a slight haze forming. It doesn’t explode or give off acrid gases, but in reactions needing pure conditions—water-sensitive synthesis or battery work—even small moisture uptake changes results. Using airtight bottles with desiccant helps. I’ve seen teams skip this, only to wonder why conductivity measurements drift.
Chemical Compatibility: Not All Reactions Go Smoothly
Handle strong acids or bases and you see some action. Concentrated hydroxides can degrade the pyridinium ring, slowly turning a clear solution murky. Acidic conditions under 25°C remain benign, but push reagents or temperature and decomposition might creep in. Compatibility shouldn’t be guessed. Data from studies shows it survives exposures to mild Lewis acids, supporting catalytic work. Still, mixing with halides—especially chloride and bromide salts—leads to ionic exchange. The unwanted byproducts often show up without warning, resulting in lost material or skewed measurements.
Best Practices for Everyday Work
Storing N-Butylpyridinium trifluoromethanesulfonate means avoiding plastic that leaches contaminants and holding back from metal tins that rust. Amber glass bottles with proper labeling help keep things in order. Use personal experience to guide shelf life. If it looks off, don’t use it. Rely on fact-based protocols, not guesswork—track opening dates, exposure times, and usage logs. Labs that treat all ionic liquids as nearly immortal risk batch-to-batch surprises that waste money, time, and credibility.
Room For Improvement—And Caution
Nobody likes throwing away expensive chemicals, but chasing purity and stability pays off in the long run. Using high-quality N-Butylpyridinium trifluoromethanesulfonate, storing it dry, and respecting thermal limits keep experiments clean. Researchers continue to hack away at stabilizers and synthesis tweaks, searching for new ways to make ionic liquids last longer under stress. Trusting results means knowing what’s in your bottle hasn’t quietly changed behind the scenes.
Bottle It Right, Keep It Safe
Anyone who’s worked in labs for a stretch knows how easy it is to lose track of storage details—one misplaced bottle, one sticky reagent, and you’re headed for headaches. Labeling gets overlooked. People use a faded marker and think a scribbled formula is enough. In reality, a legible label with the complete compound name, concentration, hazards, and the date trumps vague shorthand every time. With proper labeling, nobody’s rummaging around, wondering if a solution has expired or turned dangerous.
Keep your chemicals in sealed, original containers wherever possible. Repackaging chemicals creates extra risk: static, moisture, or a bit of dust can ruin things quickly. I’ve seen old containers swell and drip after someone figured “close enough” with the cap. Not every substance forgives mistakes—especially those that pull water out of the air, react with oxygen, or break down in sunlight. Check the Safety Data Sheet (SDS) for specifics, but dry, cool, ventilated storage works for most common compounds outside the fridge.
Forget Convenience—Think Compatibility
Even busy labs must shelve convenience for safety. It’s tempting to pile bottles of acids, bases, and organics on the same shelf. This shortcut can backfire. Sulfuric acid next to acetone invites disaster if there’s a spill or break, not because the chemicals are inherently explosive, but because fire and noxious gases easily follow accidental mixing. Spend time grouping your chemicals—acids with acids, bases with bases, oxidizers on their own route. Some institutions color-code storage cabinets for this reason. You’ll see the value if you ever try cleaning up after a minor mix-up.
Temperature, Light, And Humidity—Nothing Fancy, Just Consistent
Compounds degrade in ways that matter: too warm, and reaction rates speed up; too moist, and things can clump or react; too much light, and photosensitive chemicals break down faster than you’d expect. A dedicated fridge, set between 2°C and 8°C, goes a long way for sensitive materials. Don’t rely on a kitchen model where leftovers share space: food contamination and temperature swings ruin good research. For light-sensitive materials, amber bottles or covering shelves with blackout cloth works. Even taping foil around a bottle can extend shelf life.
Practical Handling—Protect Yourself, Protect Others
No matter your training, take compounds seriously every time. Eye protection, gloves, and a fresh lab coat beat regret. I used to think a cracked glove was no big deal. Then, a splash of strong base blew a hole through the fabric and left a nasty burn. Don’t rush; measure twice and keep pipettes clean. Small spills grow into big problems, especially with volatile or caustic chemicals. Work in a fume hood if there’s any uncertainty. It’s a minor interruption compared to explaining a lab accident to your supervisor—or a poison control officer.
Waste Disposal—Never An Afterthought
Used compounds can’t sit on a bench waiting for next week’s mystery visitor. Every substance demands a purpose-built, labeled waste container. Most institutions require documentation for pickup. Pouring anything down the drain, unless approved for that specific chemical, threatens water supplies and violates environmental law. Keep up with guidelines—even if it feels tedious—so your research, and the people around you, stay safe and on the right side of regulations.
A Close Look at the Risks
Anyone who has spent time in a chemistry lab knows the blend of anticipation and caution every time a new chemical hits the bench. N-Butylpyridinium Trifluoromethanesulfonate isn’t one of those off-the-shelf solvents you pour without thinking. This ionic liquid draws attention for its utility but quietly demands respect for the hazards hidden in its bottle.
My own encounters with pyridinium salts taught one lesson above all: never assume “liquid” means “safe.” While this compound skips the volatility of some other solvents, it introduces its own set of challenges. Exposure can irritate skin, eyes, and the respiratory system. Those warnings on the bottle aren’t just for show. If you get a bit on your hands, the discomfort speaks louder than any label. Once I brushed off a spill, thinking gloves offered enough of a barrier. Not so — irritation set in before I had the chance to rinse thoroughly.
What the Facts Say
Digging into the safety data sheets, you’ll notice recommendations for splash goggles, gloves (not the flimsy type from the supermarket), and chemical-resistant lab coats. The trifluoromethanesulfonate part hints at some of its trouble: fluorinated compounds often sting with persistence and can slip through low-grade barriers. Research from industrial safety studies, such as those referenced by the American Chemical Society, points out the importance of nitrile gloves and over-sleeves. Ventilation matters, too — prolonged inhalation can irritate airways, so working under a fume hood isn’t overkill.
Accidental heating changes the game. While N-Butylpyridinium Trifluoromethanesulfonate won’t catch fire like ether, breakdown products released under high temperatures include hazardous gases such as hydrogen fluoride. This isn’t the time to cut corners. I’ve seen teams who thought a standard open bench was enough until the acrid smell tipped off everyone that byproducts had escaped. Proper fume extraction could have spared the panic.
Solid Habits Help
Workspace organisation turns routine into protection. Designated storage locations away from incompatible chemicals—especially strong bases and acids—keep accidents rare. Emergency eyewash stations and showers shouldn’t gather dust. One day, a colleague knocked over a bottle; quick access to the shower made all the difference.
Waste disposal needs close attention. Those fluorinated residues don’t belong in the sink. Following up with hazardous waste teams prevents long-term risks to the environment and coworkers. I remember an environmental officer describing fluorine compounds in groundwater—once there, cleaning them out costs orders of magnitude more than proper lab disposal.
Solutions for Smoother Work
Training sits at the core of every safe lab. Regular refreshers on handling ionic liquids keep old hands and newcomers out of trouble. Simple steps—clear labeling, double-checking protective equipment, immediate cleanup of spills—build a culture where mistakes shrink before they become disasters. Pairing new researchers with experienced mentors helps everyone spot risks before they unfold.
N-Butylpyridinium Trifluoromethanesulfonate belongs in the set of chemicals handled with respect and preparation. Given its promise in electrochemistry and materials science, demand will only grow. As that happens, making safety part of the daily workflow ensures that innovation never comes at the expense of health.
Clarity on Purity in Everyday Products
Purity isn't just a number stamped on a label; it shapes how industries trust and handle materials. Look at common chemical ingredients found in labs and industries—from sodium chloride to acetone. Most folks see white powders or clear liquids and assume they’re all the same. Experience has taught me that small differences in composition can cost time, money, or even health. That single percentage point you see in “purity grade” could change the outcome of a manufacturing run, research result, or food product. Companies typically offer a breakdown of what to expect: technical, food, and reagent or analytical grades.
The Grades Most People Encounter
Take Sodium Chloride—plain table salt. Grocery stores never mention purity, but chemical suppliers carry food grade, typically above 99%. Build a science experiment, and you might run into lab reagent grade at 99.5% or greater. For standard cleaning jobs or de-icing, grades can dip below 97%. On paper, these all sound pure, but bits of calcium, magnesium, or even moisture can hide in the difference.
Look at Acetone used in nail polish remover and laboratory glassware washing. Industrial drums may promise 95% to 97%. Step into a laboratory, and you’ll see bottles marked 99.5% or 99.8%. High purity ensures glass comes out clean, so experiments aren’t ruined by mystery residue. For artists and mechanics, ultra-high grades only mean a higher price, not better results.
Why Purity Isn’t Just for Scientists
Everyday products, from vitamins in pharmacies to solvents in hardware stores, run on guidelines laid down by regulatory bodies. Pharmaceutical grades often hit 99% or higher because medication safety depends on tiny details. Regulations clamp down hard on impurities that could hurt people. Years ago, a friend running a supplement shop got burned when a batch didn’t meet the grade. Testing revealed impurities below 1%, but that fraction made their product illegal to sell.
Impurities can come from leftover chemicals during manufacturing, storage containers, or in some cases poor transportation. That’s where “traceability” starts to matter. Being able to show a full test report sets businesses apart and reassures people about what they’re buying. More suppliers publish Certificates of Analysis to back up their claims. Good record-keeping means fewer recalls and builds customer trust.
Solving the Problem of Impure Products
Companies serious about quality lean into better sourcing and more regular testing. Some switch to suppliers who follow Good Manufacturing Practices (GMP), which aren’t just industry buzzwords. These guidelines push for stricter controls on contamination, and every batch gets a closer look before shipping. I remember working at a university lab where a cheaper batch ruined an experiment—lesson learned. After switching to a supplier with tighter controls, we stopped having those mysterious failures.
Technology brings new tools for detection. Faster chromatography and spectroscopy let technicians spot contamination early. This boosts production and keeps bad batches from reaching shelves and labs. Folks buying for personal use often pay little notice, but those who ask for Certificates of Analysis before buying get a clearer picture of what goes in the bottle or box.
Pushing for transparency could help everyone. Open access to test results—the actual numbers—levels the playing field for small companies and keeps big players honest. It’s not flashy, but making purity part of the conversation drives smarter choices in what people use, ingest, and trust.