N-Decylimidazolium Tetrafluoroborate: Shaping the Future of Chemistry
Historical Development
The track record for ionic liquids like N-Decylimidazolium Tetrafluoroborate goes back about fifty years, back when scientists were looking to push past volatile solvents in search of smarter, less toxic options. The earliest steps happened in the ‘70s, mostly as a series of experiments in physical chemistry labs as researchers cooked up salts that stayed liquid at room temperature. Most attempts fell short because of mess, cost, or instability. N-Decylimidazolium Tetrafluoroborate found its legs only in the 1990s, as advances in organic synthesis, purification, and analytical tools finally gave chemists a handle on moisture- and air-stable ionic liquids. The arrival of this class of chemicals opened doors to more selective, tunable solvents and reaction mediums, and the recipe soon spread from specialty suppliers out to mainstream research benches.
Product Overview
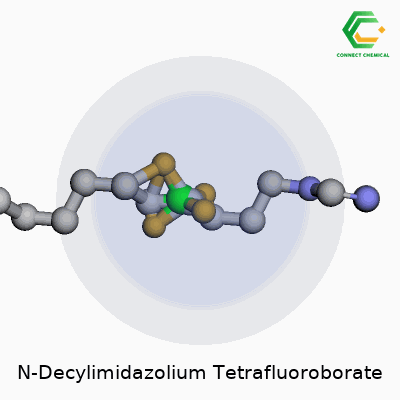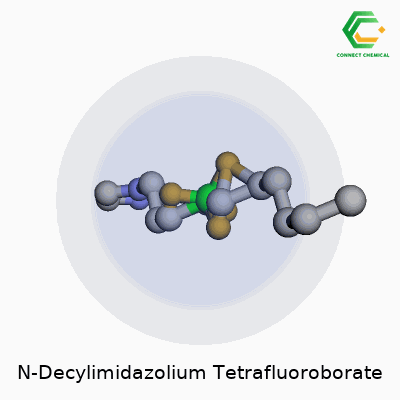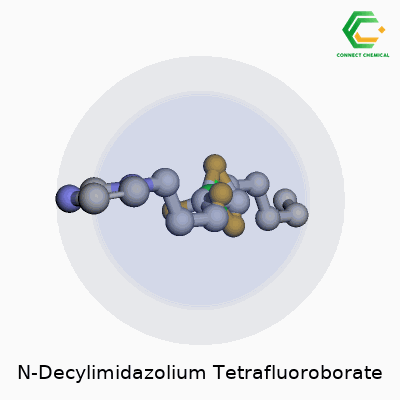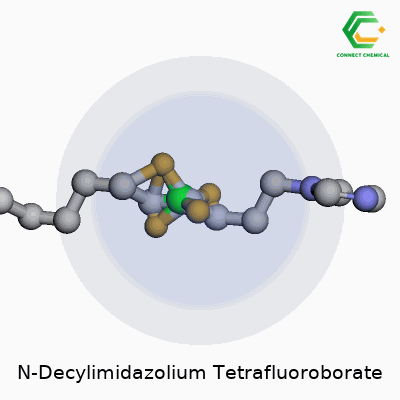
This compound—also called 1-Decyl-3-methylimidazolium tetrafluoroborate—fits into the growing family of imidazolium-based ionic liquids that people count on for both their physical properties and chemical stamina. Its structure builds off an imidazolium ring capped by a decyl chain at one end, finished up with a tetrafluoroborate anion. At room temperature, you’re looking at a clear, colorless liquid. It usually smells faintly of the starting amine—a sign of high-grade, low-impurity synthesis—not the aggressive, stinging aroma of cheap solvent blends. Users rely on its robust shelf life and a genuine consistency between batches, which keeps research and process results predictable.
Physical & Chemical Properties
N-Decylimidazolium Tetrafluoroborate stands out for a reason: barely any vapor pressure, even up above normal boiling points for standard organic solvents. The density hovers near 1.01-1.08 g/cm³ at typical lab temperatures, with a viscosity that feels heavy compared to classic alcohols or DMSO, but moves freely as it warms. The melting point sits below zero Celsius, and the liquid form stays stable well above 200°C, barring strong acids or oxidizers. The ionic liquid dissolves a surprising range of materials: it handles polar organics, some metals, and even certain biological molecules. Unlike many salts, it doesn’t draw much water from air unless the relative humidity shoots up—key for people working with moisture-sensitive reactions.
Technical Specifications & Labeling
Suppliers usually weigh out N-Decylimidazolium Tetrafluoroborate with a focus on purity—values of 97% or above are standard for research and industry orders, and most vendors provide NMR and elemental analysis certificates with shipments. Labels flag the full structure, molecular formula (C14H27BF4N2), and batch traceability. The packaging usually consists of amber-glass bottles with airtight PTFE seals, cutting light and contamination risks. The barcoding and safety tags meet GHS rules, listing hazards and storage recs right up front.
Preparation Method
Synthesis happens in two major steps. The imidazolium cation backbone forms by alkylating 1-methylimidazole with decyl chloride or decyl bromide, typically using an organic solvent under nitrogen to dodge air and moisture issues. Purity checks at this stage make the next step more reliable. The salt mixture goes through anion metathesis: the crude chloride or bromide salt gets swapped out by slow addition to a sodium tetrafluoroborate solution, with controlled temperature to avoid byproduct formation. Finished batches wash with water and undergo vacuum drying. This prep keeps decomposition, hydrolysis, or halide carryover in check.
Chemical Reactions & Modifications
One of the bright spots for N-Decylimidazolium Tetrafluoroborate comes from the ease of functional tweaking both before and after synthesis. Chemists have built analogs by modifying the side chain—using different alkyls or aromatic rings when they want special electronic or steric effects. Post-synthesis, the tetrafluoroborate anion can get traded for alternatives like PF6-, triflate, or acetate, letting scientists nudge melting points, solubilities, and reactivities. Reaction chemists use these modifications to dial in properties for a given task—whether they need to shuttle protons, stabilize intermediates, or help dissolve tough organics. Recent patents push this into tailored ionic liquid libraries, meant to fit specialized catalysis, extraction, or electrolyte niches.
Synonyms & Product Names
People in labs and supply houses call this product by several names. Most commonly, it shows up on certificates as Decylmethylimidazolium tetrafluoroborate, 1-Decyl-3-methylimidazolium tetrafluoroborate, or the code DMIM BF4. Some order lists dangle the shortened C10mim BF4 or DMIM-TFB, tying directly into the chain length. These names matter for tracking down specs or checking cross-brand equivalence because suppliers keep their stock numbers proprietary, which can confuse new buyers.
Safety & Operational Standards
No one should let the mild odor or high boiling point fool them—this is not a substance for careless use. Eyes, skin, and lungs show irritation after contact or fume inhalation; gloves (nitrile, not latex), splash goggles, and a snug fume hood keep most problems at bay. Disposal doesn’t work for the sink or trash because the compound doesn’t break down easily; most labs give it to licensed hazardous waste handlers. Heat and sparks near bulk storage cause dangerous breakdowns, especially if acid traces or oxidizers lurk nearby. Emergency planners treat bigger spills with high caution, breaking out absorbents and secondary containment. Published studies show no evidence of DNA mutation or direct carcinogenicity in standard models so far, but the absence of proof adds risk, not confidence. Safe handling and spill drills fit with OSHA and EU ReACH guidelines across universities and industry plants.
Application Area
Most users reach for N-Decylimidazolium Tetrafluoroborate as a high-performing solvent in organic synthesis and catalysis. Green chemistry circles prize it for minimizing volatile organic compounds, and electrochemists rely on its conductivity for fuel cell research and battery development. Extractive industries use it for metal recovery and rare earth processing, where traditional organics choke or degrade. Its ability to keep biocatalysts active or boost enzyme solubility has kicked off interesting projects in pharma and biotech. Researchers push boundaries with it in supercapacitor development, printable electronics, and as both a medium and additive for polymer chemistry. Each time, process demands change batch size, purity grade, or sometimes even anion choice. My own work involved using the compound as a solvent for Suzuki coupling reactions, with far better selectivity compared to DMF and reduced hazardous waste cleanup.
Research & Development
Many teams experiment with imidazolium ionic liquids every year, with hundreds of new publications filling databases. The push for sustainable chemical processes—driven by both environmental concerns and tougher laws—keeps these compounds in the spotlight. Conferences in Europe, China, and the US host whole sessions about new ligand designs, how these ionic liquids stabilize tricky catalysts, or ways to recycle precious metals from waste streams. Computational chemists feed data about structure-activity relationships into machine learning models to predict new variants, hoping to simplify scale-up for industry. Collaboration between industry and academia has paid off; new N-Decylimidazolium-based formulations have slotted into pilot plants for carbon capture, pharmaceutical intermediate crystallization, and fuel cell stack improvements.
Toxicity Research
Toxicologists dig through both cell line and environmental fate studies to figure out what happens after N-Decylimidazolium Tetrafluoroborate leaves the lab. Aquatic toxicity crops up, especially with prolonged exposure at high concentrations, leading to damage among invertebrates and plants. Biodegradation rates in freshwater and soil end up lower than ideal, which sends up red flags for widespread use, particularly outside closed-loop chemical operations. Mammalian studies show low acute toxicity through dermal and oral routes, but caution wins out because breakdown products in living systems remain underexplored. Regulatory agencies in Europe and North America have tagged the compound for further hazard evaluation. Practically, safe containment, proactive waste management, and ongoing health monitoring for frequent lab users set the standard today.
Future Prospects
Demand rises every year for cleaner, smarter alternatives to polluting solvents and specialty reactants, and N-Decylimidazolium Tetrafluoroborate fits that ticket especially well for medium- to large-scale reactors. The compound’s high thermal and chemical stability, tunable interactions, and sustainability focus make it one to watch as more industries look for ways to close recycling loops. The next phase—real leap—likely comes from pairing these ionic liquids with renewable feedstocks or integrating them into continuous manufacturing. Policymakers and green chemistry groups know the risks for aquatic life and the need for cleaner synthesis routes, so future advances must wrestle with both performance and cleanup. Chemists, engineers, and regulators work side by side on life-cycle studies and smarter disposal plans. The next decade will show how far partnerships and new discoveries can push the boundaries of what this ionic liquid class can deliver.
The Realities Behind Ionic Liquids
N-Decylimidazolium tetrafluoroborate belongs to a larger group called ionic liquids. These aren’t your everyday table salt or sugar crystals—they’re salt-based compounds that stay liquid at room temperature. Some years ago, researchers started paying attention to their unusual properties: low vapor pressure, thermal stability, and ability to dissolve lots of different chemicals. Because of this, they’ve stepped into many chemical processes, especially for those who want safer, more sustainable alternatives to old-school organic solvents.
Why N-Decylimidazolium Tetrafluoroborate Gets Attention
If you’ve ever worked in a lab or industrial plant, you’ve probably noticed the push toward “greener” chemistry. The old solvents, often flammable and toxic, bring headaches—literally and in terms of environmental regulations. N-Decylimidazolium tetrafluoroborate helps bridge that gap. It doesn’t evaporate like acetone or toluene, so air quality stays safer. In real-world settings, this means lower fire risks and less stress about breathing in nasty fumes, both for workers and for the local environment.
Actual Applications in Industry and Research
Chemists use N-Decylimidazolium tetrafluoroborate as a solvent in reactions that need both heat resistance and chemical versatility. Take catalytic reactions in organic synthesis. This compound can hold metals in solution so catalysts work more efficiently. In my experience, swapping out traditional solvents for ionic liquids sometimes bumps up yields, shaves off reaction time, or simplifies the final cleanup step.
Electrochemistry labs turn to it for making batteries or capacitors. Because it conducts electricity well and doesn’t catch fire easily, it gives engineers the freedom to design safer energy storage systems—which really matters for electric vehicles and portable devices. There’s ongoing research into using it for dye-sensitized solar cells and lithium-ion batteries, proving its value isn’t just theoretical.
Another area: extraction and separation. Refineries and pharmaceutical plants have always hunted for better ways to pull out the valuable parts from raw materials. N-Decylimidazolium tetrafluoroborate can attract certain molecules and ignore others. So industries use it to separate tricky mixtures in ways that used to require harsher chemicals.
Health, Safety, and Environmental Questions
Plenty of people want greener options, but ionic liquids aren’t perfect. Tests show that some can be toxic to fish and other life if not handled with care. While they won’t evaporate into the air, improper disposal could mean contamination in water or soil. It’s important to remember that less volatility doesn’t always mean less hazard.
People designing chemical processes or new products have a responsibility to look at the whole lifecycle: not only the ease of use and performance, but how much is needed, and what happens at the end. Responsible sourcing, collection, and recycling programs could cut the environmental risks. That’s a real challenge for manufacturers and regulators who want innovation without trading away public health.
Where Things Go From Here
As someone who’s seen plenty of trends come and go, I can say the real promise of N-Decylimidazolium tetrafluoroborate comes down to context. Used with care and a good deal of thought for safety and disposal, it opens new doors in chemistry and engineering. With the right rules in place and enough commitment to monitoring waste, it’s possible to get the best out of modern ionic liquids—without replaying the mistakes of past chemical booms.
Looking Safety Straight in the Eye
Stepping into any lab or chemical warehouse brings a beat of caution, especially with compounds like N-Decylimidazolium Tetrafluoroborate. This ionic liquid made its way into electrochemistry, solvents, and novel research projects. It draws attention because its unique chemistry pushes boundaries—on both efficiency and risk. I’ve watched gloves tinged and noses wrinkle after a spilled beaker, which always serves as a wake-up call.
Gloves That Block, Not Just Hide
Direct contact with skin can trigger rashes, itching, or far worse after longer exposure. Not all gloves are equal. Nitrile stands up better here than latex. Lab coats should cover wrists and long sleeves tuck inside gloves. I still see sleeves rolled up or folks skipping goggles for a quick pour, but chemical burns only respect vigilance—not haste.
Eye and Lung Protection
N-Decylimidazolium Tetrafluoroborate doesn’t fly out of bottles like a volatile solvent, but splashes happen in real life. Safety goggles or even full-face shields keep eyes clear. Fumes sometimes get underestimated in closed quarters. Even small amounts can irritate breathing, so using a proper fume hood isn’t just a rule—it’s a relief. When a lab skips the exhaust fan, everyone shares the stale air and increased risk.
Storage Rules Matter
Heat and sunlight change chemicals. They also draw unwanted reactions. Store this compound in tightly sealed, labeled containers out of direct sunlight and away from strong acids or bases. Keeping incompatible chemicals apart is one of those rules everyone learns fast. I’ve seen careless storage lead to ruined batches and endless cleanup.
Spill Response Built on Routine
Spills happen, and having a plan beats panic every time. Absorbent materials should be on hand, along with proper containers for waste. Never use water unless the safety sheet gives the green light—it can make things worse with ionic liquids. I’ve found that quick action, plus a solid spill protocol written in big clear letters near the bench, keeps problems small. Emergency showers and eyewash stations nearby really do save the day.
Waste Disposal: Not a Gray Area
This isn’t something that goes down the drain or into regular trash. Disposal has to follow local hazardous waste guidelines—using the right drums and documenting every step. Mixing waste streams can explode costs and risks, which frustrates not just workplace safety officers but anyone who works nearby. I’ve lost track of how many times extra attention to tracking waste saved time and money down the line.
Why E-E-A-T Deserves Respect
Google puts weight on experience, expertise, authority, and trust—those traits don’t just help with search rankings, they help keep people safe. Relying on trusted safety references, keeping current with research publications, and sharing direct experience are all habits that support strong protocols. A culture of safety starts with people who care enough to do things right, teach newcomers, and fix mistakes openly. No one learns from silence after an accident.
Smarter, Safer, Stronger Together
Working with advanced chemicals like N-Decylimidazolium Tetrafluoroborate raises the bar for everyone. Daily respect for personal protection, strict storage, real spill response, and honest waste tracking all make the difference. Out of all the labs and warehouses I’ve worked in, the safest ones grow from teams who know the risks, share knowledge, and treat every bottle with the respect it deserves.
The Basics You Need to Know
Chemistry tends to toss plenty of complicated names around, but breaking it down makes everything clearer. N-Decylimidazolium tetrafluoroborate has become a bit of a buzzword in the world of ionic liquids. These are salts that stay liquid at temperatures most folks would call “normal.” They open the door to green chemistry, fuel unique industrial processes, and help us think about solvents differently.
Chemical Formula and Structure
From an up-close perspective, this compound is actually an ionic salt made up of two parts—a cation and an anion. On the cation side, you have N-decylimidazolium. Picture imidazole, a five-membered ring with two nitrogens, linked to a decyl group, which is a ten-carbon straight chain. So, the full cation is C13H25N2+.
The anion part is tetrafluoroborate—simple enough: BF4-. Each molecule combines the advantages of the stable imidazole ring with the less reactive and fairly inert tetrafluoroborate.
Structure’s Impact on Usefulness
In my research days, the first time I ran into N-decylimidazolium tetrafluoroborate was during an attempt to swap out traditional solvents in an analytical application. The long decyl tail makes the cation more hydrophobic, changing how it mixes with water and other liquids. That property gives researchers flexibility—if you want an ionic liquid that won’t just dissolve in water, try adding a longer alkyl chain like decyl.
N-decylimidazolium pairs neatly with the tetrafluoroborate anion, which stays pretty unreactive. This combination helps avoid side reactions and brings thermal stability to the mix. For those working in electrochemistry, that matters a lot—less breakdown under heat means safer operations and a longer shelf life for your reagents.
Real-World Applications and Concerns
Industries chasing green chemistry value ionic liquids. With N-decylimidazolium tetrafluoroborate, you find an option that doesn’t give off much vapor. That’s good news for air quality inside labs and factories. In extraction processes, the structure encourages selective separation, letting you pull out chemical targets more precisely.
Yet, all of this comes with some caveats. Each new compound that hits the market deserves a review for toxicity and environmental effects. Tetrafluoroborate, while stable, can release fluoride if mishandled. Responsible practices make a difference—workers need training, monitoring, and proper disposal protocols to keep things safe.
Looking Toward Better Solutions
Some labs have talked about tuning the alkyl chain—maybe using shorter or longer versions depending on the job. As a result, you get the custom solvent with precisely the properties you want. There’s also ongoing research into more biodegradable versions. Most of my colleagues argue that combining chemical know-how with regulations—stronger oversight, open reporting, and real transparency—could push this branch of chemistry toward safer, more sustainable outcomes.
Sticking close to facts, people using N-decylimidazolium tetrafluoroborate should keep up with advances and stay in touch with research. Every new structure opens doors, but common sense needs to walk in with you.
Thinking About Chemical Safety from the Ground Up
Working in a research lab for several years, I learned quickly that storing chemicals isn’t just paperwork, it’s about real-world risks. N-Decylimidazolium tetrafluoroborate isn’t your run-of-the-mill solvent or reagent; it belongs to the family of ionic liquids, which offer fascinating properties but also call for some extra care. This compound often shows up in labs working on green chemistry, electrochemistry, or advanced materials, but anyone handling it faces a few clear responsibilities. Too many labs take shortcuts with storage, but this approach invites disaster and undercuts the innovation these chemicals make possible.
Heat, Moisture, and Light: Enemy Number One, Two, Three
The chemical makeup of N-Decylimidazolium tetrafluoroborate means it stays stable in the right conditions, but humidity turns it into a problem. Tetrafluoroborate anions react with water, sometimes forming toxic byproducts like hydrogen fluoride. I’ve seen a bench left damp ruin a pricey batch of ionic liquid overnight, which teaches a lesson you don’t forget. Labs must set up climate-controlled storage, with tightly sealed containers. Desiccators or dry boxes work well, cutting down on moisture and warding off cross-contamination. Wrapping bottles in aluminum foil or storing in opaque containers reduces light exposure, which can degrade the compound over time.
Containers, Labels, and Common Sense
Glass bottles with secure Teflon-lined caps keep unwanted air or moisture out. I remember a day when a colleague used a plastic bottle instead, arguing it was easier to handle. The cap absorbed some moisture and after a few weeks, the contents turned cloudy. Safety standards exist for a reason. It’s not just about equipment, though—labeling every container with the name, date received, and hazard information is the mark of careful work. Every chemist or technician should check labels before use. This builds trust that each step in the process started on a solid footing.
Separation from Incompatible Compounds
Simple mistakes lead to big problems. Storing N-Decylimidazolium tetrafluoroborate too close to acids or bases can trigger reactions. I once watched a near-miss get caught in a shared storage closet, where an acid spilled close to an ionic liquid. Standard practice calls for storing this compound away from anything that could react with fluorides or borates. Segregated storage lockers, chemical-proof secondary containers, and well-posted signage make all the difference. Shortcuts never pay off in chemical safety.
Training and Protocols: The Human Factor
Having the right gear in place won’t matter without well-trained people. Too often, staff turnover or lack of proper training leads to costly mistakes. Regular refreshers on chemical safety, clear operating procedures, and easily available safety data sheets (SDS) make it easier for people to do the right thing. I remember the relief in a lab where everyone followed the same checklists, so nobody had to guess how something should be stored. As more labs work with novel compounds and seek greener alternatives, these habits stop problems before they grow.
Learning from Experience
Every near-miss, ruined sample, or minor spill has a story behind it. Getting storage right for N-Decylimidazolium tetrafluoroborate keeps researchers productive, protects the workplace, and builds confidence in new chemical technologies. Trusting both the science and the habits of careful practice shapes a safer, smarter laboratory environment.
Understanding the Chemical Nature
N-Decylimidazolium tetrafluoroborate sounds technical, but it’s not just a fancy name cooked up for textbooks. This compound belongs to a family of chemicals called ionic liquids. It features a decyl (ten-carbon) chain attached to an imidazolium ring, plus a tetrafluoroborate anion. These details might seem small, but they control how the substance behaves, especially when it hits a liquid.
Looking at Water Solubility
You’d expect a simple salt to dissolve in water, but N-decylimidazolium tetrafluoroborate isn’t like table salt. That decyl tail is stubborn—it doesn’t mingle easily with water. Water loves small, charged particles that can form friendly hydrogen bonds. The long, greasy decyl chain turns that molecule into something more like oil than salt, making it dislike water. Most lab data backs this up: the more carbon you stick on the imidazolium ring, the less that salt wants to mix with water. In practice, you could stir this compound into a beaker of water all afternoon and still see a pile sitting at the bottom.
Organic Solvents Tell a Different Story
Life changes for N-decylimidazolium tetrafluoroborate when it meets organic solvents. The molecule’s oily side links up nicely with nonpolar or low-polarity liquids like dichloromethane or toluene. Researchers working with ionic liquids often see that increasing the carbon chain boosts solubility in solvents like chloroform or even ether. This lets chemists use it in extraction, separation, and special synthesis setups where water would only get in the way. In my own work, I have found ionic liquids like these to offer better control in reactions needing dry, nonaqueous conditions. They don’t evaporate like common solvents and often help stabilize sensitive reaction partners.
Why Solubility Matters in Real Experiments
Choosing the right liquid for dissolving a reagent shapes every outcome in the lab. If a scientist needs to make use of N-decylimidazolium tetrafluoroborate, they’ll want a solvent that dissolves it fully. Otherwise, the mix won’t behave as expected. Let’s say you want to use this compound to separate precious metals—using water would slow the process and lead to frustration. Swapping in an organic solvent where the salt truly dissolves makes things flow smoothly. Time saved, resources managed better, and cleaner results every step of the way.
Supporting Data and Real-World Results
Multiple journal articles show solubility data for imidazolium salts. For similar salts with long chains, water solubility drops to minimal values, while organic solvents welcome them. It’s even true for mixtures—some researchers mix small amounts into polar organic solvents like acetonitrile or methanol, finding useful levels of solubility, though not as high as in more hydrophobic media. Real results beat theory—no one wants to waste money on a liter of solvent that leaves half their chemical stuck undissolved in a flask. Facts from the European Chemicals Agency and peer-reviewed papers back up this performance, so there’s no magic involved.
Switching Gears: Problem-Solving
Labs that work with ionic liquids sometimes hit a wall due to tricky solubility. One clear solution involves tweaking the chain length or changing the solvent system. A careful match between chemistry and solvent turns sticky, stubborn solids into workable solutions. Sometimes, chemists apply gentle warming or ultrasonic baths if a little extra push is needed. But forcing dissolution in water rarely pays off for this compound. For projects needing water compatibility, switching to shorter-chain ionic liquids or different anions can help. Focusing on the structure-solubility link saves frustration and cash.
Science, Safety, and Better Results
Solubility impacts safety as well. Proper solutions reduce handling risks, keep reactions contained, and lower waste. By looking at facts and chemical properties, researchers use N-decylimidazolium tetrafluoroborate where it works best. This keeps science practical and efficient, turning theoretical knowledge into safer, more reliable work.