N-Dodecylimidazolium Tetrafluoroborate: The Story and the Science
Historical Development
Long before the world chased “green” solvents, chemists searched for a better way to carry out reactions that typically demanded harsh conditions or environmentally questionable ingredients. N-Dodecylimidazolium tetrafluoroborate made early appearances in the late 1990s, when ionic liquids appeared on the radar as safer and more customizable alternatives to volatile organic solvents. Over the last two decades, researchers built a toolkit around imidazolium-based ionic liquids like this one, with long carbon chains and varied counterions. The idea grew familiar in research circles: swap metal-heavy or flammable liquids for salts that flow like oils at room temperature. N-dodecylimidazolium tetrafluoroborate stood out thanks to its balance of hydrophobicity from the dodecyl chain and the chemical stability provided by the tetrafluoroborate ion.
Product Overview
Some chemicals immediately call to mind a narrow field of use. This one bucks that trend. On the bench, N-dodecylimidazolium tetrafluoroborate comes as an almost colorless to pale yellow viscous liquid or sometimes a waxy solid, depending on storage temperature. Its low vapor pressure solves many laboratory safety headaches, and chemists like its flexibility: it blends without difficulty into other polar and nonpolar solvents and rarely causes surprises in multi-component mixtures. The combination of a large organic tail and a delocalized imidazolium cation yields a salt that refuses to crystallize in most conditions. For ionic liquids, that point—staying liquid—opens the possibility for new applications in catalysis, extraction, electrochemistry, and materials science.
Physical & Chemical Properties
You notice quickly that this compound does not act like table salt or like benzene, despite featuring simple elements. The melting point depends on the precise water content and storage history but falls below room temperature except under very dry conditions. Viscosity stays notably higher than water or traditional organic solvents, and the surface feels oily or even soapy. Solubility trends in polar and nonpolar environments fascinate scientists: the imidazolium core brings some water compatibility, but twelve carbons in the dodecyl group pull it firmly into the realm of oils and hydrocarbons. Tetrafluoroborate supplies stability in the face of many nucleophiles and electrophiles but runs into trouble with strong acids or bases, which can unleash hazardous decomposition. For technicians, electrical conductivity stands out. Ionic liquids like this conduct current quite well, though not on the level of molten salts. These properties carve space for this material in numbing electrochemical research and advanced green synthesis.
Technical Specifications & Labeling
Handling this chemical in the lab, most containers carry standardized labels: chemical name, molecular formula (C15H29BF4N2), and the familiar hazard pictograms, if provided commercially. Purity matters—small differences in water content or leftover synthesis byproducts can shift melting points or change color—so suppliers specify purity above 98%. Quality control batches measure water content, melting point, and check for halide or acid contaminants, since these subtle impurities can thwart sensitive applications. The correct UN number, safety labels, and storage instructions keep compliance officers happy and protect staff. For storage, an amber glass bottle with a tight seal protects the contents from ambient moisture and contamination.
Preparation Method
Ionic liquids in this family almost always start from an alkylation reaction. For n-dodecylimidazolium tetrafluoroborate, production typically involves coupling 1-dodecyl bromide or chloride with 1-methylimidazole under mild heating, forming the dodecylimidazolium halide. That intermediate then undergoes anion metathesis by adding a solution of sodium tetrafluoroborate, precipitating the halide salt and freeing the tetrafluoroborate as the counterion. The final product requires thoughtful purification—thorough extraction, removal of transferred halide impurities, drying over anhydrous magnesium sulfate or similar agents, and filtration. Some labs go further, recrystallizing or distilling under vacuum to achieve cleaner material for electrochemical work. Everyone involved in the process traditionally needs care and patience: a single misstep in drying can tack on days lost to troubleshooting later.
Chemical Reactions & Modifications
N-dodecylimidazolium-based ionic liquids open doors for more modifications than the average salt. Swapping the counterion customizes solubility and reactivity; converting to hexafluorophosphate, triflate, or other anions tunes the ionic liquid for different tasks. Chemists sometimes click new functional groups onto the dodecyl tail or tweak the imidazole ring to craft surfactants or catalysts precisely set for a given application. In practical settings, researchers often use these salts as inert partners in biphasic reactions or as solvents that stabilize unusual intermediates. Advanced work explores polymerizing the imidazolium unit for use in membranes or as part of new conductive plastics for batteries and fuel cells. The sheer versatility makes this family a frequent choice for exploratory chemistry, where standard solvents give limiting, unchangeable results.
Synonyms & Product Names
You may find this compound under a handful of names—1-dodecyl-3-methylimidazolium tetrafluoroborate, C12MIM BF4, or [C12MIM][BF4]. Researchers often abbreviate to save time, but chemical suppliers list full IUPAC names to meet regulatory and safety standards. Checking the fine print on a supplier’s datasheet reveals small variations in naming: some drop the “methyl,” some keep it. For those digging into patents or journal archives, quick synonym checking spares hours of fruitless searching.
Safety & Operational Standards
The story of ionic liquids in industry doesn’t leave safety behind—handling them with clean technique prevents accidents. N-dodecylimidazolium tetrafluoroborate shuns volatility, so fire risk drops compared to lighter hydrocarbon solvents. Nevertheless, nothing replaces strict adherence to lab safety protocol: gloves, goggles, and fume hood when handling quantities above a few milliliters. Prolonged skin contact brings the risk of irritation; ingestion or inhalation of aerosols should not be risked. Chemical disposal guidance usually insists on hazardous waste collection due to uncertain environmental breakdown. Quality control labs require regular calibration of equipment since impurities or variable water content can lead to product inconsistency or failed batch releases. Staff training, proper storage, and MSDS consultation help keep standards on the right track.
Application Area
The reach of these ionic liquids keeps spreading. In my experience, colleagues in electrochemistry rely on them for stable, conductive media to test cutting-edge batteries and capacitors. Analytical chemists mine their ability to dissolve both polar and nonpolar compounds, improving extraction efficiency in real-world environmental samples. Catalysis researchers tweak their formulation to create unique reaction conditions for coupling or hydrogenation reactions traditionally viewed as too risky or slow. The long dodecyl chain makes this specific liquid effective as a surfactant or phase-transfer catalyst in systems with both aqueous and organic phases. Green chemistry fans use it to swap out volatile, toxic alternatives, making pilot plants a bit safer. If one visits the patent literature, N-dodecylimidazolium derivatives appear in anti-static coatings, pharmaceutical formulations, lubricant additives, and even waste remediation.
Research & Development
Anybody knee-deep in R&D knows the pain of solvents that force a process into one narrow lane. This compound’s story shines especially for scientists seeking new media with designer properties. Ongoing studies explore tuning ionic conductivity, thermal stability, and breakdown behavior. Research teams now probe deeper—looking at microstructure in solution, the way local organization changes reactivity, or how to anchor large biomolecules for separation and purification. Universities with strong materials science programs push the envelope by embedding ionic liquids like this one into polymers, gels, and membranes to shape next-generation electronics, flexible screens, and medical sensors. Conferences buzz with discussions about enhancing selectivity, resisting fouling, or recovering precious metals using imidazolium-based solvents. The direct feedback from these studies loops back into product improvement: stronger, cleaner, and cheaper batches for commercial rollout.
Toxicity Research
I have seen the shift from “non-volatile means non-toxic” to a much more nuanced approach to lab safety. Early publications praised ionic liquids’ lack of flammability and evaporation, but toxicity testing paints a more complex picture. The dodecyl chain brings excellent solvency but also stirs concern about aquatic toxicity, persistence, and possible bioaccumulation. Toxicity data on fish, algae, and invertebrates suggests that longer alkyl chains and certain anions (including tetrafluoroborate) show higher aquatic toxicity than short-chain or more biodegradable ionic liquids. Human cell assays tend to show low acute cytotoxicity, but the data remains limited. As regulations tighten around persistent organic pollutants, full life-cycle testing now stands as a baseline requirement.
Future Prospects
Nobody expects innovation to slow down here. From my perspective and that of the industry at large, the prospects look wide open. Synthesis techniques will surely refine further—reducing waste, streamlining purification, and aiming for greener starting materials. The push for biodegradable ionic liquids could spark a new wave of structural modifications, perhaps replacing the dodecyl chain with renewable or enzymatically cleavable groups. New fields—CO2 capture, advanced separations, and nano-material fabrication—stand to benefit from materials that offer control at the molecular level. The regulatory landscape influences progress too; transparent toxicity and degradability data will shape commercial wins and losses. For scientists and entrepreneurs willing to invest the effort, N-dodecylimidazolium tetrafluoroborate tells an ongoing story—one of adaptability, challenge, and real potential in chemistry’s search for safer, smarter solutions.
What Makes N-Dodecylimidazolium Tetrafluoroborate Stand Out
N-Dodecylimidazolium tetrafluoroborate sounds like a mouthful, but the uses for compounds like this reach beyond chemistry labs into places most people never consider. As an ionic liquid, it steps up where water, oil, or other solvents struggle. Most folks won't spot it on a shelf, but it runs quietly behind the scenes in a growing number of applications.
Green Solvents for Chemical Reactions
Chemists have wrestled with finding solvents that do their job without hurting the environment or people working with them. Traditional solvents often bring a risk of fire or toxicity, filling the air with fumes and causing headaches for safety officers. N-Dodecylimidazolium tetrafluoroborate gives industry a safer alternative. Its low volatility and stability make it handy for reactions that demand heat or special care. This matters in pharmaceuticals, where purity and safety are non-negotiable. The push toward “green chemistry” keeps ionic liquids relevant, especially as governments and consumers keep a close eye on pollution and safety.
Electrochemistry and Battery Research
Energy storage pops up in every tech conversation, from cars to smartphones. Most batteries chase longer life or greater storage without bursting into flames, especially with the rise of renewable energy. N-Dodecylimidazolium tetrafluoroborate comes up as a component in electrolyte research for batteries and supercapacitors. Its ability to stay stable at high voltages, resist leaking, and hold up over many cycles sets it apart from older liquid electrolytes. Experiments in labs explore how it could help lithium or sodium batteries last longer or offer a safer package—so fewer fires, fewer recalls.
Extraction and Separation
Refineries and manufacturers need to pick out key chemicals from a mix without wasting time or material. This job often chews through gallons of water or releases waste streams nobody wants. N-Dodecylimidazolium tetrafluoroborate performs well as an extracting agent for metals, dyes, and organic compounds. Its structure lets chemists tailor separation processes to pick up even trace amounts of material. You see its value in recycling electronic waste, where rare metals hide inside discarded circuit boards, or in water treatment for taking out stubborn contaminants.
Catalysis and Organic Synthesis
Speeding up chemical reactions with less mess makes industry more efficient. Catalysts based on N-Dodecylimidazolium tetrafluoroborate can boost reaction rates while keeping byproducts at bay. Its use stretches into fine chemical and material synthesis, including custom pharmaceuticals or electronics materials. In my own work with specialty polymers, I’ve seen how these ionic liquids offer a tool to push reactions that water or toluene can’t handle, especially in sensitive or multi-step syntheses. The outcome is cleaner chemistry and sometimes higher yields, which saves money and reduces waste.
Pushing Toward Better Solutions
None of these advancements come without cost. Ionic liquids require careful disposal, and their synthesis can demand rare starting materials. But their benefit grows as industries search for methods that conserve energy, protect workers, and squeeze more value out of each reaction. If the field can make production cheaper and lifecycle management cleaner, these chemicals will grow in influence, especially in newer technology and environmental cleanup.
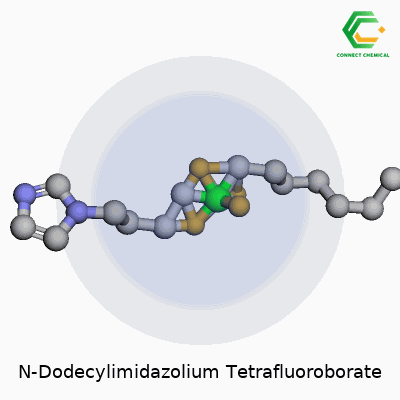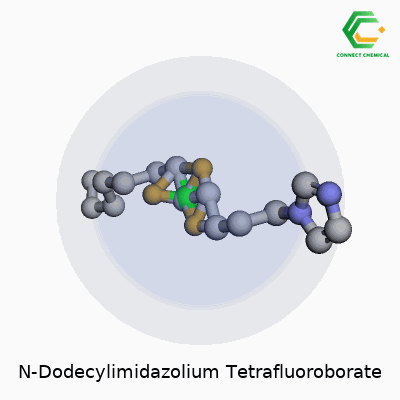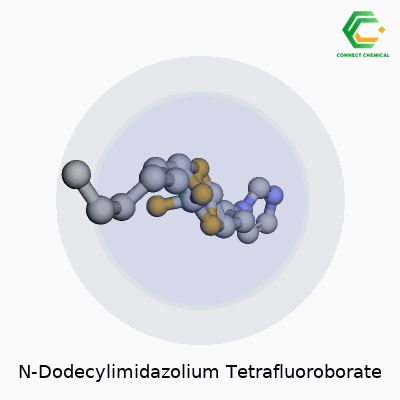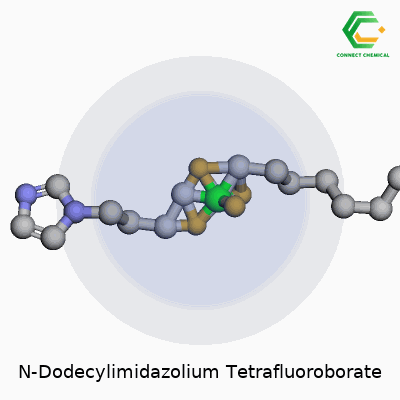
Chemical Structure of N-Dodecylimidazolium Tetrafluoroborate
Looking at this compound, what stands out is its two-part makeup. On one side, you find the N-dodecylimidazolium cation. This comes from an imidazole ring—a five-membered aromatic containing two nitrogens—linked to a dodecyl group, a straight carbon chain that runs twelve atoms long. This chain gives the molecule distinct amphiphilic qualities. The tail does more than stretch out the molecule; it affects solubility, enables interactions with non-polar substances, and brings some surfactant properties, which opens up the door for interesting chemical applications.
On the other side, the tetrafluoroborate anion enters the scene. Chemical shorthand calls it BF4-. It sits here tightly packed, with four fluorine atoms anchored to a central boron atom. The BF4- part helps with stability and plays a significant role in the ionic liquid family. Given how ionic liquids have drawn attention for their role as green solvents, the structure and size of both the cation and anion matter just as much as their function.
Molecular Weight: Getting Specific
Molecular weight gives researchers, chemical engineers, and even students a concrete number to rely on when designing synthesis or handling materials. For N-dodecylimidazolium tetrafluoroborate, this takes some tallying up. The formula for the cation part looks like this: C15H29N2+. Add to that the tetrafluoroborate anion, BF4-. With straight calculation, the numbers build up:
- Carbon: 12.01 × 15 = 180.15
- Hydrogen: 1.01 × 29 = 29.29
- Nitrogen: 14.01 × 2 = 28.02
- Boron: 10.81 × 1 = 10.81
- Fluorine: 19.00 × 4 = 76.00
Tally the total: 324.46 g/mol (cation) + 86.81 g/mol (anion) leads to a full molecular weight right around 411.27 g/mol.
Importance and Practical Use
Reading up on ionic liquids over the past decade, the industry keeps coming back to N-dodecylimidazolium tetrafluoroborate’s unique profile. The long alkyl chain improves solubility in organic solvents and can even form micelles, right in the middle of a chemical reaction. In my lab days, we used related ionic liquids to replace volatile organic solvents, especially for challenging syntheses where temperature control or non-flammability made a difference. Working with something like this cuts down on harmful emissions and offers a safer bench experience. Publications across the Royal Society of Chemistry and the American Chemical Society have shown its role in electrochemistry—especially for stable electrolyte systems used in energy storage devices.
Consider the case of green chemistry. The less-toxic, more recyclable the solvent, the greater the chance of broad adoption. But wider industry usage always runs into hurdles. Availability at scale, cost, and long-term toxicity all influence how fast these chemicals find their way outside of academic studies. Making the case for further research into both shorter- and longer-chain versions might reveal safer or cheaper variants, especially if made from renewable feedstocks instead of petroleum-derived chains.
Future Pathways: Research, Regulation, and Responsible Use
N-dodecylimidazolium tetrafluoroborate continues to sit near the forefront of ionic liquid development. Its structure, with a hydrophobic chain and a robust anion, puts it right in line with current research needs for more sustainable solvents and advanced materials. Yet, thorough environmental and human health assessments still need broader support. Community-driven science and open research networks can speed up such studies, while clearer guidelines from regulatory bodies will ensure that the benefits of switching to ionic liquids don’t come at hidden costs down the line.
Structured understanding of chemical properties, coupled with responsible, fact-based adoption, shapes whether a compound like this carves out a big role in tomorrow’s labs and industries.
Understanding the Chemical
N-Dodecylimidazolium Tetrafluoroborate stands out in a laboratory or industrial setting due to its role as an ionic liquid. The substance looks unassuming — usually a viscous liquid that doesn’t shout danger. Despite the surface calm, it demands respect. I’ve worked with chemicals that slip past your caution because they don’t give off strong odors or burn your fingers right away. This is one of those. Its boron-fluorine component signals hidden hazard, especially if you let moisture creep in.
Storage Guidelines Matter
This chemical won’t keep itself fresh. I always set aside a dedicated spot for ionic liquids, far from acids, bases, and oxidizers, because even one careless moment can trigger an unwanted reaction. The storage zone needs toughness. Polypropylene containers work much better than glass, which can etch if the tetrafluoroborate anion leaches fluoride ions. A tightly sealed lid forms the front line — not just to keep the air out, but to block water vapor, since hydrolysis slowly releases corrosive byproducts and degrades the salt.
Routine checks matter. Once, I saw a bottle sweating white crystals near the cap: a real sign that the chemical had reacted with humidity in the air. Keeping everything under a dry nitrogen atmosphere saves headaches, especially in climates with sticky summers. Desiccators or glove boxes with active drying agents serve as insurance. No one likes to open a container, expecting ready-to-use liquid, and find half of it crusted over.
Personal Safety Takes Priority
The structure of N-Dodecylimidazolium Tetrafluoroborate means you don’t want it on your skin. Irritation, in my experience, doesn’t wait long — gloves matter. Nitrile or neoprene gloves cut risks, along with goggles and a buttoned-up lab coat. Splashes sneak up fast, even with slow-pour liquids. I’ve seen colleagues shrug off a tiny spill, only to deal with rash or persistent itch hours later.
Some routines help more than fancy equipment. A designated fume hood keeps vapors and accidental splashes away from shared workspaces. Wet handling multiplies hazards, so equipment stays dry, and tools don’t jump from one task to another without cleaning. A friend once found cross-contamination as the cause of a failed experiment and a mild chemical burn. Simple labeling, careful workflow, and never reusing pipette tips between bottles keep it all in order.
Emergency Planning Counts
Emergencies don’t give warnings. Even with double-layer gloves and steady hands, mistakes happen. Familiarity with the lab’s eyewash and safety shower comes in handy. I run through spill drills a couple of times a year because real accidents don’t let you check instructions mid-crisis. Quick cleanup with absorbent, non-reactive materials saves a lot of trouble. Waste picks up in sealed polyethylene containers, never down the drain, not even the smallest drops. Local laws usually forbid that for a good reason: tetrafluoroborate salts linger in the water table and cause lasting harm.
Experienced Habits Make the Difference
I’ve watched new team members grow confident enough to stop giving N-Dodecylimidazolium Tetrafluoroborate its due. That’s how mistakes spark fires and shut down operations. Real safety means routine respect for every step — dry storage, proper PPE, fume hoods, spill kits, and a backup plan. That’s not just good science, it’s respect for yourself and your coworkers.
Understanding the Character of N-Dodecylimidazolium Tetrafluoroborate
N-Dodecylimidazolium tetrafluoroborate belongs to a family of ionic liquids where an imidazolium ring joins with a long alkyl chain and a tetrafluoroborate anion. In labs and various industrial setups, solubility speaks louder than many other physical properties. People working with ionic liquids like this one want straightforward answers: will it dissolve in water, in organic solvents, or both? The answers impact how scientists, product formulators, and engineers use this compound in catalysis, separations, extraction, green chemistry, or even electronics.
Water Solubility: More Chain, Less Mix
Imidazolium salts with short alkyl chains nearly always mix with water easily, but as the alkyl chain stretches out—like the dodecyl (12-carbon) chain—water solubility takes a nosedive. Literature and hands-on experience both show that N-dodecylimidazolium tetrafluoroborate struggles to dissolve in water. The long hydrocarbon tail acts like a waterproof coat, pushing the molecule out of aqueous environments. Some references confirm this by reporting either very limited water solubility or plain insolubility for similar structures.
Organic Solvents: Finding the Right Fit
On the flip side, that same nonpolar tail sticks like a magnet to organic solvents. N-dodecylimidazolium tetrafluoroborate often dissolves in solvents like chloroform, dichloromethane, or even acetone. The more nonpolar the solvent, the more likely this ionic liquid will blend right in. Shelling out funds on trial and error can frustrate chemists, but in practice, reaching for organic solvents with moderate polarity usually brings success with longer-chain imidazolium cations.
Why Solubility Choices Matter in Research and Production
Solubility don’t just fill up columns in a textbook. A solvent mismatch can scrap whole syntheses or drive up separation costs. Ionic liquids designed to work as catalysts or extraction agents often rely on separating cleanly from water or sticking to organic layers. If someone tries to use N-dodecylimidazolium tetrafluoroborate as the active ingredient for a water-based process, they’ll probably hit a wall—or, worse, waste time and money before realizing it won’t cooperate. In separation processes, though, its reluctance to mix with water but affinity for organics might actually turn into an advantage.
Addressing Challenges and Seeking Solutions
If water compatibility is a dealbreaker, scientists might swap out the dodecyl for a shorter chain, or add polyether or sulfonate side chains to push the molecule toward greater hydrophilicity. Some labs also mix in co-solvents or tweak salt concentrations to trick the compound into dissolving, but these strategies hinge on the final use and purity required.
On the regulatory side, people often ask about the environmental profile. The tetrafluoroborate anion earned a modestly stable reputation, but high concentrations or poor disposal may still raise eyebrows—especially in water systems—so careful containment and waste management make sense. Both researchers and production line teams benefit from checking the latest studies, as solubility can shift a process from lab curiosity to scalable solution—or just as often, vice versa.
Conclusions Beyond the Data Table
Solubility never boils down to yes or no. Long-chain imidazolium salts like N-dodecylimidazolium tetrafluoroborate lean hard toward organic solvents and back away from water, and this reality shapes what’s possible in the lab and factory. The best outcomes come from clear-eyed planning, smart solvent choices, and an openness to reworking the molecule itself if a particular solution simply won’t mix.
Understanding Purity in the Lab
Any chemist who’s worked with ionic liquids knows that purity isn’t just a box to tick—it’s the line between clean data plots and hours wasted hunting down mysterious side reactions. N-Dodecylimidazolium Tetrafluoroborate doesn’t break the trend. High-purity chemical reagents matter because even tiny contaminants can throw off sensitive reactions or introduce new, unplanned pathways. Purity usually sits at 98% or even higher, and that makes a real difference. I’ve seen well-designed experiments get sabotaged by a percent or two of impurity, so I don’t take listed specs lightly. For this compound, reliable vendors often guarantee 98% or 99% minimum purity, with some going even higher for special batches.
The path to confirming a batch’s quality runs through techniques like NMR, HPLC, and ion chromatography. Think of these not as sales talk but as insurance. NMR gives insight into the structure, confirming that the imidazolium ring and dodecyl chain are intact. HPLC picks out possible organic byproducts left over from synthesis. Ion chromatography chases after anion purity, ensuring the tetrafluoroborate part isn’t hiding some stray halide. If you’ve ever switched suppliers because a bottle of ionic liquid didn’t quite behave as expected, you know how critical these checks become.
Practicality in Package Sizes
Package size doesn’t seem like an exciting detail—until you buy too much or too little. Most suppliers understand that N-Dodecylimidazolium Tetrafluoroborate taps into both research and industrial projects, so they offer everything from 1-gram vials up to 100-gram or even kilogram bottles. In an academic lab, that 5-gram jar usually stretches far enough for a dozen trials, while a production team might burn through kilograms in a day.
There’s another point here I’ve picked up firsthand: packaging matters for purity too. Small, air-tight glass bottles keep out the moisture and air that can degrade sensitive ionic liquids. Oversized packages invite risk, especially if storage practices slip and atmospheric water slowly seeps in. That single bottle on a warm shelf in a humid lab can turn a reliable ionic liquid into a source of unexpected variables.
Why This Matters Beyond the Bench
Purity and packaging choices flow downstream. When electrochemists try to squeeze out better battery efficiencies, or when green chemists search for cleaner alternatives to traditional solvents, they trust that the bottle’s label matches the contents inside. If a project scales up, inconsistent lots or variable purity won’t just hurt the data—they’ll multiply costs fast. Honestly, over the years, I’ve seen countless meetings drag on because a supplier couldn’t deliver the same quality twice in a row.
A simple approach to these problems keeps projects on track: question the spec sheets, run in-house checks, and choose packaging that matches real consumption needs. Don’t chase bulk discounts just for the savings if purity drops or shelf-life suffers. Reliable chemistry starts before you uncap the bottle.